
The search for greener ethylene
Making the basic chemical produces substantial carbon emissions. Chemical companies are trying to change that
One of the pillars holding up the chemical industry rests on shaky ground. That pillar, ethylene, is arguably the most important petrochemical raw material. Its handy carbon-carbon double bond is easily opened and made accessible for countless chemical reactions.
For instance, free-radical polymerization of ethylene creates the molecule’s largest-volume derivative, polyethylene, which is used in plastic bags and packaging. The reaction of ethylene with chlorine produces ethylene dichloride, a precursor to another plastic, polyvinyl chloride. Combined with oxygen, ethylene becomes ethylene oxide for detergents and ethylene glycol.
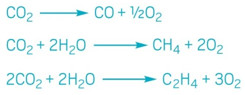
The electrochemical reduction of carbon dioxide has the potential to transform waste CO2
into a number of useful molecules, including ethylene.
But forming that convenient double bond uses a lot of energy. Cracking the hydrogens off an ethane molecule or longer hydrocarbons like naphtha requires temperatures in excess of 800 °C. And the heat for the enormous furnaces where cracking takes place comes from combustion of natural gas or other fossil fuels.
According to EcoCatalytic, which is developing an alternative route to ethylene, conventional cracking generates roughly 1.5 metric tons (t) of carbon dioxide for every metric ton of ethylene produced. Globally, that amounts to more than 260 million t of CO2 emissions per year. This is almost 0.8% of the world’s total carbon emissions, which were estimated at 34 billion t for 2020, according to the Global Carbon Project, an organization tracking climate change.
Chemical makers have long been aware of ethylene’s carbon problem. Back in the 1980s and ’90s, they explored routes such as oxidative coupling of methane and dehydration of biobased ethanol to reduce the energy intensity of making ethylene. But commercial success was modest, and companies continued to invest billions in conventional ethylene capacity.
Now, facing heightened public pressure to reduce emissions, chemical makers are redoubling their efforts to strip carbon from ethylene production. Many have corporate pledges to cut carbon output. For instance, both Dow and Shell aim to be carbon neutral by 2050. BASF wants to keep carbon dioxide emissions flat, even as its business grows, through the remainder of this decade.
Firms like these see cracking—a big source of their emissions today—as a potential well to draw carbon savings from. They are testing multiple new technologies, including electrifying ethylene furnaces, making ethylene directly from COsub>2, and using catalytic routes to ethylene. But commercial processes, experts say, aren’t likely to appear until the end of the decade.
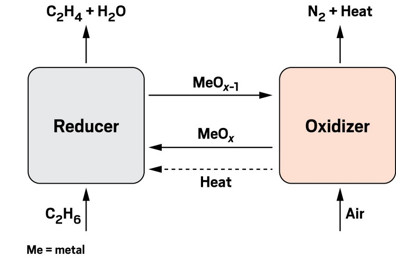
EcoCatalytic's oxidative looping process uses oxygen from a metal oxide to transform ethane into ethylene and water.
Ethylene is one of the three largest COsub>2 emitters in the chemical industry; the other two are propylene and ammonia, according to John Murphy, president of the Catalyst Group, a consulting firm. “You certainly go after the ones where you have the biggest bang for the buck,” he says.
Edward Stones, global business director for energy and climate change at Dow, says about 50% of the company’s CO2 emissions come from process heat sources such as cracker furnaces. “Our technologies range in intensity,” he says. “And I would say that steam crackers are among the higher ones. ”Last June, Dow and Shell announced a collaboration to develop an “e-cracker.” The idea is to harness electricity from renewable sources to generate the heat needed for cracking, much like using an electric stove in place of a gas range.
“As we look at electrification and what electrification could bring to us, we think at some of our sites it could reduce emissions by as much as 50%,” says Manav Lahoti, global sustainability director for hydrocarbons at Dow.
Lahoti says the technological effort is focused on issues such as designing the heating element and understanding how electrical heating would affect different feedstocks, some of which require more heat to crack than others.
Dow and Shell are not alone in developing an electric furnace. In 2019, BASF, Borealis, BP, LyondellBasell Industries, Sabic, and Total announced they would pool their efforts into the Cracker of the Future Consortium.
That same year, BASF disclosed that it is collaborating with the engineering firm Linde on electric furnace technology. At the time, the company said it was working on issues such as managing voltage to keep workers out of danger. The company said it was poised to test elements that operated at about 1,000 °C.
A BASF official now says the company is “finalizing the basic engineering documents with our partners and preparing the decision for further steps, like an investment in a pilot plant.”As important as Rivaling the technical hurdles is the challenge of the electrical grid itself, Dow’s Stones says. Supplies of renewable energy can be fickle, as they often depend on the weather. But consistency is critical for industrial plants. “It’s very difficult to overcome intermittency,” he says. “Long term, our goals are contingent on competitive, affordable, and sustainable energy of all types.”
The Finnish start-up Coolbrook has its own spin on electrified cracking. But in its case, the heat isn’t generated by running current through a resistor or an electric arc. Instead, Coolbrook generates heat from kinetic energy.
At the heart of Coolbrook’s Roto Dynamic Reactor is a rotor that revolves at 20,000 rpm, or about 300 m/s. It pushes naphtha or ethane into a diffuser chamber at supersonic speeds, creating shock waves that heat the feedstock to cracking temperatures.
CEO Harri Johannesdahl says Coolbrook’s technology offers shorter residence time than conventional crackers, leading to ethylene yields that are up to 30% higher. He says the process could reduce the overall energy consumption of an ethylene cracker complex by 30% and would be emissions-free with a renewable energy source.
Coolbrook was founded in 2012 with the technology of Vladimir Bushuev, a veteran scientist in the Soviet Union’s space program. The company recently signed a collaboration with the University of Cambridge.
Coolbrook plans to open a pilot plant in Geleen, the Netherlands, by the end of this year with 400 kg/h of capacity. Johannesdahl says the company is already in discussions with chemical companies about the next step for the process: a pilot plant that would have 5,000–10,000 kg/h of output.
“Definitely we are ahead of everybody else,” Johannesdahl says, comparing Coolbrook’s progress to companies working on electric cracking.
The Catalyst Group’s Murphy recalls similar approaches over the years. For example, more than a decade ago, ExxonMobil teamed with Pratt & Whitney Rocketdyne to employ rocket-engine design to move gases quickly. It’s hard to tell how Coolbrook’s technology will pan out, he says. “You certainly have the standard challenges in that it is not extremely well known at scale,” he says.
Also relatively untested is another strategy for reducing CO2 emissions: producing ethylene from CO2 itself.
In December, the Brazilian petrochemical maker Braskem announced a research partnership with the University of Illinois at Chicago (UIC) to develop a route to ethylene based on the electrochemical reduction of CO2. The aim is to use the CO2 coming from the flue gas of conventional ethylene furnaces to make more ethylene.
Braskem is no stranger to green ethylene. It has been making ethylene from sugarcanederived ethanol in Brazil for a decade, using it for its I’m Green polyethylene product line.
Scientists have long studied the electrochemical reduction of CO2 into hydrocarbons and oxygen-containing chemicals like formic acid. Efforts intensified around 2010, says Meenesh Singh, a chemical engineering professor at UIC who is the point person on the project with Braskem. “This is a very well-investigated area,” he says.
In the electrochemical system, hydrogen ions are generated from water at the anode, and CO2is reduced at the cathode until carbons and hydrogens combine into ethylene. In a commercial system, making a kilogram of ethylene will require 15–30 kW·h of electricity, Singh estimates. Deployed at full scale and using renewable power, the system could reduce the carbon intensity of making ethylene by 20–30%.
A number of challenges remain, however. One, Singh says, is perfecting the composition and structure of the copper-based catalyst for long-term stability and activity. Another is separating the CO2 and ethylene that intermingle in the reactor. An additional challenge is concentrating CO2 from cracker waste gas, an area where the UIC researchers already have patents.
UIC and Braskem hope their collaboration will yield a prototype. After that, Singh and collaborators aim to develop a pilot plant that would run for about 5 years.
Berkeley, California–based start-up Opus 12 is also pursuing the electrochemical reduction of CO2, but it is looking into a number of products besides ethylene, including carbon monoxide and methane.
Opus 12’s first target is carbon monoxide, according to CEO Nicholas Flanders. He explains that it doesn’t take as much energy to reduce CO2 into CO as it does to make ethylene. Because the reaction is less demanding, Opus 12 can spend more time on catalyst development. Flanders also anticipates demand from specialty chemical makers interested in onsite production of carbon monoxide for syntheses.
“It makes sense for us to develop and achieve all the initial scaling with a molecule that customers need at small and intermediate scales and then have done all of that learning to address a market like ethylene,” Flanders says.
Opus 12 is working on ethylene with the Spanish energy and chemical producer Repsol. Opus 12 has also received a Small Business Innovation Research grant from NASA for this work, with an eye toward one day making polyethylene from the CO2 of Mars’s atmosphere.
Opus 12 is trying to hone the selectivity of its catalysts to minimize side products. It also seeks to improve efficiency. If the company could get within 50% of the theoretical efficiency of 13 kW·h per kilogram of ethylene, the process “would get pretty interesting from an economic perspective,” Flanders says.
The cost, at current industrial electricity rates, would be about $1,000 per metric ton of ethylene, Flanders says. That’s about four times the cost of conventional ethylene cracking in the US, although it is roughly in line with recent global selling prices of ethylene. But he points out that every metric ton of ethylene produced would capture about 3 t of CO2. “If you are using low-carbon electricity, that’s a pretty big impact,” he says.
So far, Opus 12 has raised $20 million from government grants and private investors. Flanders acknowledges that the company is “at least a couple of years out from having something at industrial scale.”
Chris Dziedziak, project manager for the Catalyst Group, cautions that the potential for side products is a significant obstacle for electrochemical reduction. In addition to making separations more challenging, by-products can rapidly degrade the catalyst.
Electric cracker furnaces are “probably more near term, in our view, than electrochemical CO2conversion on a large scale,” Dziedziak says.
Another approach to reducing CO2 emissions from ethylene production fits right inside the chemical industry’s wheelhouse: new routes to ethylene that apply catalysis or chemical looping to conventional ethane feedstock.
Dow is working on catalytic ethane dehydrogenation technology modeled on its new fluidized catalytic dehydrogenation (FCDh) process for converting propane into propylene. The company claims that the propane technology can reduce energy consumption by 25% versus conventional propane dehydrogenation.
The company will install a demonstration unit with 100,000 t of annual propylene capacity at its Plaquemine, Louisiana, complex by the middle of next year. Dow has already licensed the technology to the US propylene producer PetroLogistics.
Dow’s Lahoti says its propylene technology is similar to fluidized catalytic cracking, which refineries use to break long-chain hydrocarbons into gasoline and propylene. Such units operate at lower temperature and pressure than conventional propane dehydrogenation units, he says. Additionally, the catalysts Dow is using have higher selectivity than in conventional propane units.
Applying the same platform to ethylene could reduce emissions by 40–50%, Lahoti says. But Dow scientists have some challenges ahead. “We are talking about a completely different chemistry,” he says. “So there is a lot of work that has to be done on the catalyst development side and also how we modify the current FCDh technology from a process standpoint.”
In another catalytic approach, Shell, Dow’s partner on electrified crackers, announced in October that it is working with Linde on ethane oxidative dehydrogenation. Linde already runs a demonstration plant in Pullach, Germany, using its version of the technology, which it calls Edhox.
In the process, ethane and oxygen react to form ethylene and water. It runs below 400 °C and can reduce CO2 emissions by as much as 60%. A potential disadvantage of the technology is that it produces a lot of acetic acid, a sideline that might not be appealing to every ethylene producer.
EcoCatalytic is developing another oxidative process, this one involving looping of a metal oxygen donor. The Woburn, Massachusetts–based firm says it has the potential to reduce CO2 emissions from ethylene production by 80%.
In EcoCatalytic’s process, an oxygen from a metal oxide reacts with ethane to form ethylene and water. The metal flows back to an oxidizer-regeneration unit to pick up another oxygen. CEO John A. Sofranko compares the role of the metal to hemoglobin, which carries oxygen around the bloodstream.
The energy for the reaction comes from the oxidation of the metal. So far, EcoCatalytic has been working primarily with manganese. “The process generates its own heat, and no furnaces are required,” Sofranko says.
The company received a $5.5 million grant from the US Department of Energy’s Advanced Research Projects Agency–Energy program and $2 million from the DOE’s Advanced Manufacturing Office. EcoCatalytic put the money toward a pilot plant at Southwest Research Institute in San Antonio.
The pilot has already racked up more than 1,000 h of operation, Sofranko says. Key challenges will be managing heat and fluid dynamics. He admits that it might be many years before the process is ready. “We are going to need to build another scale-up from this pilot way before we commercialize,” he says.
EcoCatalytic isn’t seeking venture capital funding but is on the hunt for a chemical company to work with. “What we have been doing is looking for strategic investors who can help us finance our next step forward,” Sofranko says.
The Catalyst Group’s Murphy warns that looping processes, which have reactive components that get separated, recycled, and returned, add components and costs. “Typically, there’s some reason why the larger companies haven’t funded those sorts of approaches,” he says.
But for large chemical companies, the time has come for big thinking, audacious bets, and the resolve to see projects through, Dow’s Lahoti says. “These are whizbang developments,” he says. “To get to that 2050 carbonneutrality goal, incremental solutions are not going to get us there. You need major change, major innovation, and that is what we are working on.”
How to make styrene production greener
Redox catalyst makes monomer from ethylbenzene without any added steam
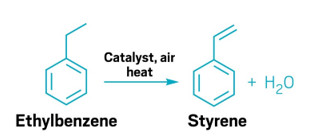
A redox catalyst could make the oxidative dehydrogenation of ethylben-zene to styrene more energy efficient.
Industry analysts estimate that by 2023, chemical makers will produce about 30 million metric tons of styrene—a monomer that’s used to make polystyrene and copolymers. Now, chemical engineers report a way to make styrene that could save energy and significantly reduce CO2 emissions associated with its production (Nat. Commun. 2021, DOI: 10.1038/s41467-021-21374-2).
The process, developed by North Carolina State University’s Fanxing Li and coworkers, relies on a redox catalyst with a mixed calcium manganese oxide core surrounded by a potassium ferrite shell. In addition to spurring the oxidative dehydrogenation of ethylbenzene to styrene, the material serves a source of oxygen for the reaction, which also produces water. Purging the catalyst with air replenishes its oxygen.
The current industrial route to making styrene from ethylbenzene has a typical single-pass yield of 54%. It requires high temperatures as well as steam, which provides heat and pushes the reaction’s equilibrium toward styrene. The process developed by Li’s team has a 91% yield. And though it does require high temperatures, it doesn’t need any steam, and it generates heat. As a result, the process requires 82% less energy and emits 79% less CO2 than the industrial route. Li says that while the process—which he has patented—works well in a laboratory, his team still needs to work on scaling it up for large reactors.
“The production of styrene is among the least energy efficient large-scale chemical processes,” says T. Brent Gunnoe, an expert in catalysis at the University of Virginia, in an email. The work from Li’s team, he says, is “an important demonstration of how new approaches to catalyst design can potentially lead to increased energy efficiency.”
Solvent molecules catalyze surface reactions
Study identifies new role played by organic solvents in reactions at solid-liquid interface
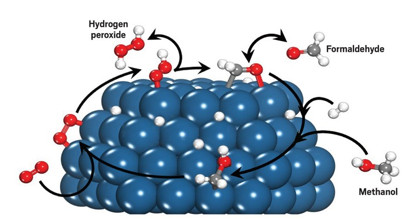
Hydroxymethyl species formed from methanol or formaldehyde mediate redox reactions
that convert oxygen to hydrogen peroxide. Pd = blue, O = red, C = gray, H = white.
Solvents are known to play key roles in solution-phase catalytic chemistry. For example, a good solvent can boost reaction rates by increasing the solubility and mass transfer of reagents relative to a different solvent. Much less is known, however, about the role solvents play in reactions at the solid-liquid interface.
In a new study focusing on that scenario, researchers find that organic solvent molecules can bind to the surfaces of metal nanoparticles and spontaneously form species that mediate redox reactions (Science 2021, DOI: 10.1126/science.abc1339). The findings may lead to ways of increasing reaction rates and product selectivities and to reducing the volume of organic-solvent waste.
The work grew from efforts aimed at understanding recent observations showing that molecular hydrogen and oxygen react in methanol and other solutions at palladium nanoparticle surfaces to form hydrogen peroxide. Manufacturers make some 5 million metric tons of H2O2 annually, mainly via an energy-intensive process with anthraquinone as the starting material. Directly reacting hydrogen and oxygen gases could save energy, but the reaction is tough to control and leads mainly to water, which is ther-modynamically favored relative to H2O2.
To figure out what’s going on, a team led by David W. Flaherty of the University of Illinois at Urbana-Champaign and Matthew Neurock of the University of Minnesota examined the solid-liquid interface by combining kinetic isotope measurements, spectroscopy, and computational techniques.
The group found that liquid-phase methanol molecules bind to Pd, forming stable hydroxymethyl intermediates. These species readily transfer electrons and protons to adsorbed oxygen, forming H2O2 and formaldehyde. Formaldehyde then oxidizes hydrogen, regenerating the hydroxymethyl species, which form more H2O2. Pure water doesn’t drive the reaction because water molecules don’t transfer electrons efficiently. However, the team showed that this catalysis does happen when small amounts of methanol or formaldehyde are dissolved in water, suggesting a strategy for reducing industrial organic-solvent waste.
According to catalysis specialist Lars C. Grabow of the University of Houston, surface chemists typically think of solvents in terms of how they stabilize surface intermediates. This team takes the concept a step further, he says, showing that the solvent can serve as a cocatalyst. Grabow notes that the study reveals how these species form on surfaces and that characterizing reactions catalyzed in this way will create opportunities for researchers to further improve reaction rates and selectivity.
Chemical & Engineering News