

“Катализ: сбалансированное использование ископаемых
и возобновляемых ресурсов”
(30 августа – 4 сентября 2015 г., Казань, Россия)
http://www.europacat2015.com
В этом году Европейский конгресс по катализу (EuropaCat XII) впервые проходил в России – в г. Казань с 30 августа по 4 сентября. Конгресс был посвящен проблемам сбалансированного использования ископаемых и возобновляемых ресурсов.
Повышенный интерес к актуальной тематике крупнейшего в Европе каталитического форума позволил собрать в Казани 837 участников из 44 стран мира. Среди организаций с наиболее многочисленными делегациями можно отметить Институт катализа СО РАН (108 участников), Казанский (Приволжский) федеральный университет (22), Казанский национальный исследовательский технологический университет (15), Fritz-Haber-Institut der Max-Planck-Gesellschaft, Germany (12), Xiamen University, China (10), UCCS-CNRS, France (8), Eindhoven University of Technology, The Netherlands (8), Technical University of Munich, Germany (8).
Организаторами Конгресса выступили: Российская академия наук; Федеральное агентство научных организаций, Москва; Министерство образования и науки Российской Федерации, Москва; НП “Национальное каталитическое общество”, Москва; Правительство Республики Татарстан, Казань; Академия Наук Республики Татарстан, Казань; Казанский научный центр РАН, Казань; Институт катализа им. Г.К. Борескова Сибирского отделения РАН, Новосибирск; Институт органической химии им. Н.Д. Зелинского РАН, Москва; Московский государственный университет имени М.В. Ломоносова, Москва; Институт органической и физической химии имени А.Е. Арбузова Каз НЦ РАН; ОАО “Татнефтехиминвест-холдинг”, Казань; Казанский национальный исследовательский технологический университет, Казань; Казанский (Приволжский) федеральный университет, Казань; Республиканское химическое общество им. Д.И. Менделеева Республики Татарстан, Казань; ООО “Мономакс”, Санкт-Петербург. Конгресс EuropaCat XII проводился под патронажем Президента Республики Татарстан Р.Н. Минниханова.
Финансовую поддержку Конгрессу оказал Российский фонд фундаментальных исследований. Партнерами, спонсирующими Конгресс, выступили следующие компании: ПАО “Татнефть”, ПАО “Таиф-НК”, ПАО “Нижнекамскнефтехим”, ПАО “Казаньоргсинтез”, ОАО “ГАЗПРОМ НЕФТЬ”, CAS SciFinder, ПАО “СИБУР Холдинг”, SPECS Surface Nano Analysis GmbH, ООО “Сервис-центр “ХромоСиб”, ООО “Новомичуринский катализаторный завод”, ООО “Брукер”, ООО “Промэнерголаб”.
В рамках Конгресса состоялись XI Международный симпозиум “Селективное окисление как ключ к повышению ценности новых ресурсов” и Международный симпозиум “Образование в области катализа”, привлекший солидную аудиторию молодых ученых. Во время работы Конгресса прошла выставка спонсоров, собравшая 22 отечественные компании и зарубежные фирмы.
Брошюра с Научной программой Конгресса объемом 520 стр. (включая аннотации докладов, каталог Конгресс-выставки, рекламу спонсоров и организаторов) и сборник тезисов на флеш-картах объемом 2184 стр. были предоставлены участникам при регистрации. Электронному изданию сборника тезисов присвоены Международный стандартный книжный номер (ISBN 978-5-906376-10-7) и номер Госрегистрации (0321504175).
Материалы статей наиболее интересных докладов Конгресса будут опубликованы в специальных номерах журналов Topics in Catalysis и Catalysis Today.
В заключительном выступлении Президент EFCATS проф. Йоганнес Лерхер подчеркнул высокий научно-организационный уровень конгресса EuropaCat, впервые прошедшего в России.
В научную программу XII Европейского конгресса по катализу были включены 8 пленарных лекций, 21 ключевая лекция, 225 устных (20 мин) и 106 кратких устных докладов, а также около 700 стендовых презентаций. На Конгрессе обсуждались наиболее важные области катализа:
Работа Конгресса проводилась одновременно на четырех устных сессиях. 700 стендовых презентаций были разделены на две постерные сессии; кроме того, из числа постеров были отобраны 106 кратких устных сообщений для представления на 11 тематических симпозиумах.
Пленарные лекции
Лекция профессора Ананикова (V.P. Ananikov, Россия) была посвящена различным каталитическим реакциям органического синтеза, в первую очередь процессам с образованием С-С связей. Автор сообщил, что типичные реакции Сузуки-сочетания часто протекают не на металлокомплексных палладиевых катализаторах, а на присутствующих в системе наночастицах и кластерах палладия. Были предложены некоторые необычные для гомогенного катализа концепции, такие как концепция адаптивных каталитических систем и органического синтеза “с атомным разрешением”.
Профессор Лерхер (J. Lercher, Германия) в своем пленарном докладе рассказал об изучении каталитически активных центров и закономерностей протекания ряда каталитических процессов в ограниченном пространстве пор цеолитов. Проанализировано влияние строения активных центров, размера пор и их химического окружения на выход и селективность изучаемых реакций.
 Prof., Dr. Johannes Lercher
Prof., Dr. Johannes Lercher
Michel Boudart Plenary Lecture (2015)
Пленарная лекция профессора Беллуси (G. Bellussi, Италия) была посвящена разработке новых видов жидких синтетических топлив. Были рассмотрены экономические, технологические и социальные аспекты производства биотоплива в различных странах.
В докладе профессора Хатчингса (G. Hutchings, Великобритания) подробно рассмотрено текущее состояние исследований в области применения нанесенных катализаторов на основе наночастиц золота, а также биметаллических (Au-Pd) наночастиц для различных процессов органического синтеза. В частности, обсуждались промышленно значимые реакции гидрохлорирования ацетилена, окисления спиртов, активации С-Н связей, прямого каталитического синтеза пероксида водорода. Любопытно, что добавки платины к биметаллическим Au-Pd наночастицам позволили повысить селективность окисления бензилового спирта за счет подавления образования побочного продукта – толуола.
Пленарная лекция профессора Хаппа (J.T. Hupp, США) была посвящена исследованию свойств мезопористых координационных каркасов (MOF) и возможностей их применения в качестве одноцентровых гетерогенных катализаторов. Рассматривались как общие методические вопросы (требования к пористости, стабильности, строению активных центров потенциальных катализаторов), так и экспериментальные результаты по катализу ряда химических реакций на пористых координационных каркасах. Показано, что для исследования механизмов катализа на MOF могут успешно применяться квантово-химические методы расчета.
Профессор Шлегль (R. Schlögl, Германия) рассказал об in situ исследованиях активных центров гетерогенных катализаторов, в том числе катализаторов с так называемыми “динамическими” активными центрами. Подобная ситуация может иметь место, когда твердая поверхность катализаторов подвергается существенной перестройке в ходе каталитической реакции, что не позволяет четко охарактеризовать динамические системы с помощью классических понятий (например, каталитическая активность).
Высочайший уровень современных исследований продемонстрировал профессор Фройнд (H.-J. Freund, Германия), рассказавший в своей пленарной лекции о синтезе и детальном исследовании на атомарном уровне сложных модельных катализаторов на основе тонких оксидных пленок и 2D-цеолитов.
 Prof., Dr. Hans-Joachim Freund
Prof., Dr. Hans-Joachim Freund
François Gault Lectureship Award (2013)
Доклад профессора Скоглунда (M. Skoglund, Швеция) имел большую практическую направленность. Автор подробно рассказал о фундаментальных исследованиях катализаторов на основе различных цеолитов, применяемых для селективного восстановления оксидов азота в условиях избытка кислорода.
Ключевые лекции
Ключевая лекция доктора Хелвига (S. Helveg, Дания) была посвящена современным аспектам электронной микроскопии. Автор подробно рассказал о возможностях применения просвечивающей электронной микроскопии высокого разрешения, позволяющей детально изучать активные центры катализаторов с атомарным разрешением, а также о тенденциях развития данного метода.
Тему фундаментальных исследований продолжил в своей лекции профессор Нейман (K.M. Neyman, Испания), известный специалист в области квантово-химических исследований. Были представлены новые оригинальные результаты расчетов структуры и каталитических свойств моно- и биметаллических кластеров металлов.
Профессор МакИндой (J.S. McIndoe, Канада) рассказал о современных тенденциях в исследовании каталитических реакций комплексом взаимодополняющих методов, позволяющих детально исследовать химические процессы на поверхности катализатора в реальном времени.
Профессор Ченти (G. Centi, Италия) в своем докладе сообщил о современном состоянии солнечной энергетики для производства альтернативных видов топлива. Были подробно освещены две основные каталитические реакции, имитирующие фотосинтез, – фотокаталитическое получение водорода и фотокаталитическое восстановление углекислого газа с образованием метана под действием солнечного света.
Сходной по тематике была ключевая лекция профессора Кан Ли (C. Li, Китай), в которой были подробно рассмотрены катализаторы и механизм полного фотокаталитического разложения воды под действием видимого излучения, а также особенности фотоэлектрохимического получения электрического тока. Работы группы проф. Ли отличаются использованием современных уникальных приборов для характеризации, что позволяет получать достоверные данные по активному компоненту фотокатализаторов и, безусловно, делает его доклад очень полезным и информативным для исследователей в области фотокатализа.
Проблеме синтеза активных фотокатализаторов для полного разложения воды была посвящена и ключевая лекция профессора Таканабе (K. Takanabe, Саудовская Аравия). В своей лекции проф. Таканабе описал многоступенчатый процесс создания активных фотокатализаторов, в результате которого можно получить материалы с высокой чувствительностью к видимому свету, эффективным разделением зарядов, удельной каталитической активностью и разветвленной макроструктурой.
Большой интерес вызвала ключевая лекция профессора Руппрехтера (G. Rupprechter, Австрия) по новым in situ и operando методам исследования катализаторов перспективных процессов паровой конверсии метанола и метана, окисления угарного газа и фотокаталитического разложения воды.
В лекции профессора Джонса (C. Jones, США) была сделана краткая оценка возможностей применения нанесенных катализаторов в химических производствах. Предложено семейство диродиевых катализаторов, нанесенных на пористый силикагель, для различных реакций С-С сочетания.
Профессор Кениг (B. König, Германия) рассказал о перспективах разработки реакций окислительного С-С сочетания с применением фотокатализаторов, использующих энергию видимого света. Существующие примеры позволяют говорить о возможности использования фотокатализаторов видимой области для процессов фотоокисления, окислительного С-С сочетания, арилирования.
Использование никелевых катализаторов для процессов Кумада-сочетания и прямого алкилирования алкинов и гетероциклических соединений обсуждалось в работе профессора Ху (X. Hu, Швейцария); обсуждался также ряд аспектов механизма катализа данных процессов, универсальных для различных типов реакций сочетания.
Профессор Геворгян (V. Gevorgyan, США) сделал обзор своих работ за последние 15 лет, посвященных развитию каталитических методов образования различных гетероциклов из алкенов и алкинов в присутствии металлокомплексных катализаторов на основе Au, Ag, Cu. Отмечено, что большинство из обсуждаемых реакций протекает через стабилизированные металлом карбеновые интермедиаты.
В ключевой лекции профессора Дескорме (C. Descorme, Франция) был дан анализ новых подходов к очистке высококонцентрированных токсичных водных стоков, которые не могут быть очищены традиционными биологическими методами. Доклад основан как на детальном анализе современной литературы по тематике, так и на обзоре работ докладчика. Рассмотрено использование методов гидродегалогенирования и каталитического восстановления. При этом основное внимание было уделено комплексу проблем, которые возникают при очистке высококонцентрированных стоков. Было также рассмотрено использование окислительных процессов с участием гидроксильных радикалов как активных интермедиатов процесса. Показано, что использование данного подхода позволяет удалять наиболее “проблемные” компоненты из влажных воздушных потоков.
Лекция профессора Парвулеску (V. Parvulesku, Румыния) была посвящена использованию кислотно-основных (Льюисовых) катализаторов для синтеза ценных химических продуктов тонкого органического синтеза. В первой части лекции были рассмотрены монометаллические и сплавные нанесенные катализаторы, активными центрами которых являются наночастицы металлов. Были проанализированы различные методы приготовления катализаторов, в которых металлический компонент обладает выраженной льюисовской кислотностью. При этом особое внимание было уделено влиянию кислотно-основных свойств на селективность в различных реакциях органической химии. Во второй части лекции были рассмотрены нанокатализаторы, обладающие свойствами основания Льюиса, для приготовления которых был использован гидролиз Mg(OCH3)2. Эффект кислотности Льюиса был исследован в целом ряде процессов, включая гидрирование двойной С-С связи, гидрогенолиз сложных молекул, стереоселективное гидрирование связи С-О в присутствии двойной С-С связи. Влияние льюисовской основности было продемонстрировано на примере реакций трансэтерификации.
Лекция профессора Лейтнера (W. Leitner, Германия) была посвящена использованию СО2 как для производства продуктов тонкой химии, так и в процессах энергетики. В лекции были рассмотрены катализаторы и каталитические процессы для селективной конверсии СО2 в муравьиную кислоту и высшие карбоновые кислоты, получения метанола с использованием СО2 в качестве исходного соединения, а также синтеза полиуретана. Было убедительно показано, что СО2 может представлять перспективный С1-блок для получения топлив, полимеров, а также продуктов тонкой химии и фармацевтики.
В своей лекции профессор Роман-Лешков (Yu. Roman-Leshkov, США) рассмотрел ряд вопросов, связанных с использованием возобновляемых ресурсов, а также конверсии биомассы на цеолитных катализаторах обладающих высокой льюисовской кислотностью. Особое внимание было уделено каталитическим процессам, которые могут быть проведены на катализаторах Sn-Beta. Было показано, что цеолитные катализаторы, обладающие высокой льюисовской кислотностью, могут с успехом использоваться в целом ряде каталитических реакций образования новых С-С связей на основе возобновляемых субстратов, что может оказать существенное влияние на общую концепцию использования биомассы в процессах синтеза самых разнообразных химических соединений.
Основной темой лекции профессора Ольсбе (U. Olsbye (Норвегия) было использование нового класса нанопористых материалов, таких как MOF (metal-organic frameworks) в реакциях олигомеризации этилена и восстановления СО2. Были представлены методика синтеза и анализ физико-химических и каталитических свойств Zr-MOF систем, разработанных в Университете Осло. Показано, что полученный материал обладает высокой стабильностью и механической прочностью.
В ключевой лекции профессора Татсуми (T. Tatsumi, Япония) был проведен детальный анализ целого ряда процессов, которые позволяют использовать альтернативные ресурсы вместо традиционной нефти. Было показано, что цеолитный катализатор, разработанный японскими учеными, дает возможность селективно конвертировать метанол в пропилен и бутены. Интересный процесс трансформации глюкозы в гидроксиметилфурфурол на катализаторах типа БЕТА был также разработан в лаборатории автора. В лекции были рассмотрены вопросы утилизации СО2 с использованием источников возобновляемой энергии и вопросы фотокаталитического разложения воды под воздействием света видимого диапазона.
В своей ключевой лекции профессор Нам (I.-S. Nam, Ю. Корея) дал детальный анализ ситуации в области селективного каталитического восстановления (СКВ) оксидов азота с использованием мочевины в качестве восстанавливающего агента. Были детально проанализированы преимущества и недостатки таких каталитических систем как традиционный V2O5/TiO2, а также нового поколения катализаторов на основе цеолитов (ZSM-5, SSZ-13, Beta и т.д.) Было показано, что использование современных физико-химических методов (ЭПР, XANES и др.) позволяет с высокой степенью точности определить строение активных центров катализаторов СКВ, что приводит к созданию новых высокоактивных, селективных и стабильных катализаторов СКВ, которые могут использоваться для удаления оксидов азота из выхлопных газов как стационарных, так и мобильных источников (в первую очередь автомобилей, оснащенных дизельными двигателями).
Ключевая лекция профессора Ходакова (A. Khodakov, Франция) была посвящена созданию высокоэффективного бифункционального катализатора прямого синтеза диметилового эфира из синтез-газа. Катализатор на основе медного компонента и цеолита ZSM-5 с иерархической структурой позволяет осуществлять синтез диметилового эфира в одну стадию; при этом, как было показано в лекции, катализатор обладает высокой активностью и стабильностью.
В своей ключевой лекции профессор Васалос (I.A. Vasalos, Греция) рассматривал вопросы, связанные с использованием цеолитных катализаторов для облагораживания моторных топлив, получаемых из возобновляемых ресурсов в ходе процессов Фишера-Тропша, гидрирования растительных масел, а также термическим и каталитическим пиролизом биомассы. Большая часть лекции была посвящена использованию цеолита ZSM-5 для модификации процесса каталитического пиролиза биомассы, в котором ZSM-5 играет роль как катализатора, так и компонента, переносящего тепло при проведении процесса в реакторе с циркулирующим кипящим слоем.
В лекции профессора Караханова (E. Karakhanov, Россия) были рассмотрены вопросы использования катализаторов, содержащих в качестве активного компонента наночастицы металла, инкапсулированные в структуру различных носителей. Катализаторы были приготовлены с использованием различных типов мезопористых гибридных материалов, а их каталитические свойства исследованы в реакциях селективного и полного гидрирования ароматических и ацетиленовых соединений.
Устные доклады
Секцию № 1 “Новые каталитические материалы и процессы для надежного обеспечения сырьевыми материалами” можно, в известном смысле, назвать ключевой в работе Конгресса, так как именно на ней предполагалось рассмотреть и обсудить основные инновации в области создания катализаторов и каталитических процессов, что должно было выявить определенные тенденции в развитии науки о катализе и каталитических технологий. В соответствии с тематикой секции, представленные устные доклады отражали два основных направления – создание новых каталитических материалов и новые каталитические процессы.
Работа, представленная Г.В. Вангом (Шанхай, Китай), была посвящена синтезу цеолитных материалов слоистого строения, в которых кремний-органические фрагменты располагаются между слоями кристаллического цеолита со структурой MWW. Показано, что полученные материалы обладают высокой стабильностью как при прокаливании, так и в ходе реакции получения кумола алкилированием бензола пропиленом.
Новым цеолитным материалам был посвящен доклад, представленный А. Астафаном (Страсбург, Франция). В нем обсуждались материалы, имеющие сложную морфологию. Показаны возможности повышения их устойчивости к закоксовыванию в реакциях образования углеводородов из этанола.
Многоуровневые структуры, названные “нанореакторами”, были рассмотрены в докладе А. Симакова (Энсенада, Мексика). При декорировании частиц золота (“ядро нанореактора”) церием или палладием удалось повысить их каталитическую активность в отношении процессов восстановления нитрофенолов до аминофенолов и окисления СО.
Концепции “нанореактора” был также посвящен доклад, представленный В. Ордомским (Лилль, Франция). В данном случае эта концепция реализуется на примере частиц кобальта, находящихся внутри сфер, образованных пористым оксидом кремния. Проведение процесса синтеза Фишера-Тропша в присутствии таких катализаторов позволяет сузить молекулярно-массовое распределение продуктов и сдвинуть его максимум в область соединений С13-С15.
Структуры, также имеющие металлическое “ядро” (Au), но в оболочке из пористого материала (цеолиты различных типов) рассмотрены в докладе С. Кегнеса (Люнгбю, Дания). Работа, представленная П. Пандисом (Халкис, Греция), посвящена синтезу и характеризации Al,Fe-замещенных силикатов лантана со структурой апатита и исследованию каталитических свойств нанесенных на них Ni-содержащих систем в реакции паровой конверсии глицерина.
Смешанные NiMo-системы, нанесенные на активированный уголь (АУ) и промотированные калием, как катализаторы гидрирования CO до высших спиртов – предмет исследования, представленный Е. Хераклеос (Салоники, Греция). Показано, что сочетание использования АУ в качестве носителя и предварительной кислотной обработки предшественника активной формы катализатора является условием достижения его оптимальных свойств с точки зрения выхода целевого продукта. В докладе Р. Ченчера (Осло, Норвегия) рассмотрены результаты работ по созданию высокостабильного и селективного катализатора дегидрирования пропана, не содержащего хрома.
Работа Р. МакВикер (Кардифф, Великобритания) была посвящена селективному окислению метана (под давлением 30 атм) пероксидом водорода в водной среде при 50°С в присутствии Au-Pd катализатора. Автор считает, что контролируемый синтез катализатора, ведущий к получению биметаллических частиц с узким распределением по размерам с максимумом около 3 нм, позволяет добиться высокой селективности по сумме метанола и метилгидропероксида, выше 90%.
Л. Фраталоччи (Милан, Италия) рассмотрела возможную позитивную роль транспортных ограничений в кобальтовых катализаторах “корочкового” (“egg-shell”) типа для интенсификации процесса в реакторах синтеза Фишера-Тропша. Искажение кинетики превращения СО за счет диффузионных ограничений может приводить к выигрышу в выходе соединений С25+ при проведении синтеза Фишера-Тропша в реакторах со стационарным слоем катализатора.
В докладе, представленном П. Бенито (Болонья, Италия) рассматривается получение высокостабильного покрытия частицами родия пенометаллического носителя на основе фехраля (Fe-Cr-Al сплав). Оптимизация каталитических свойств в реакции парциального окисления метана до СО и Н2 достигается за счет варьирования содержания родия, которое определяется составом раствора при электрохимическом синтезе покрытия-предшественника.
Р. Хенри (Осло, Норвегия) использовал различные спектральные методы, квантово-химические расчеты, микрокинетическое моделирование и традиционные каталитические эксперименты для раскрытия механизма процесса олигомеризации этилена в присутствии Ni-содержащих цеолитов и сделал вывод о сходстве этого процесса с аналогичными реакциями роста углеводородной цепи, известными в гомогенном катализе.
Группа представленных работ была посвящена процессам гидродесульфирования и гидродеоксигенации (ГДО) молекул, рассматриваемых как сырье для компонентов моторных топлив ископаемого и природного происхождения. Так, в работе В. Когана (Москва, Россия) исследована эволюция и межслоевая динамика активных центров катализаторов на основе промотированных сульфидов переходных металлов в присутствии водорода и в инертной среде в ходе десульфирования, гидродесульфирования и гидрирования нефтепродуктов, синтезов на основе СО и Н2 и конверсии этанола в присутствии водорода.
Ввиду все возрастающих требований к моторным топливам актуален поиск альтернативных гидрообессериванию процессов. В работе, представленной З.Р. Исмагиловым (Новосибирск, Кемерово, Россия), проанализирована возможность сочетания традиционной очистки от серы с процессами окислительного обессеривания. Показана техническая и экономическая привлекательность сочетания этих двух подходов.
О необычном использовании традиционных катализаторов гидрообессеривания было рассказано в докладе Г.А. Бухтияровой (Новосибирск, Россия). Показано, что нанесенные сульфиды Mo, Ni-Mo и Co-Mo могут быть эффективными катализаторами гидроконверсии сложных эфиров, в том числе компонентов растительных масел.
И. Хорачек (Литвинов, Чешская Республика) также представил доклад, посвященный использованию сульфидных катализаторов для получения сырья для нефтеперерабатывающих предприятий из возобновляемых источников (масла органического происхождения и триглицериды из различных отходов).
В работе М. Перони с соавторами (Мюнхен, Германия) для гидродеоксигенации пальмитиновой кислоты использованы фосфиды W, Mo и Ni.
Еще одна группа работ может быть охарактеризована как каталитический синтез на основе компонентов возобновляемого сырья, в том числе растительного происхождения.
В работе С. Ордонеза с соавторами (Овьедо, Испания) сравниваются реакционные способности альдегидов, выделяемых из биомассы (фурфураля и гидроксиметилфурфураля, ГМФ) в реакции альдольной конденсации с ацетоном в присутствии смешанных оксидных (MgZr и MgAl) катализаторов.
П. Маки-Арвела с соавторами (Турку, Финляндия) представили работу, посвященную получению поверхностно-активных веществ на основе соединений, выделяемых из водорослей, а именно, ацилированию амино-спиртов жирными кислотами на цеолите бета. Синтезу гваифенезина (отхаркивающее средство и препарат для лечения заболеваний верхних дыхательных путей, простой эфир глицерина и гваякола – 2-метоксифенола) из возобновляемых источников сырья (глицерина и гваякола – продукта переработки лигнина) посвящена работа, представленная Г.Д. Ядавом (Митунга, Индия). Показано, что в ряду исследованных катализаторов оптимальным с точки зрения сочетания активности, селективности и стабильности является прокаленный гидротальцит.
Управление селективностью в процессе получения диолов (в первую очередь С3-продуктов) из целлюлозы на Ni-Zn-катализаторах, синтезированных на основе углеродных нанотрубок, – предмет исследования К. ван дер Вийста с соавторами (Тондхейм, Норвегия). Показано, что целевые соединения являются продуктами превращения фруктозы как реакционноспособного интермедиата.
Кинетика и механизм процесса ГДО и дегидрокарбоксилирования-конденсации C5-кислот, получаемых из биологического сырья, в компоненты моторных топлив исследованы в работе, доложенной И.Л. Симаковой (Новосибирск, Россия). Описаны пути оптимизации процесса для получения выхода целевых продуктов выше 80%.
В докладе А. Чистякова (Москва, Россия) представлены данные о конверсии этанола в присутствии Au-Ni и Au-Cu катализаторов.
В работе Д. Отюской с соавторами (Гент, Бельгия) с использованием микрокинетического подхода проанализирована селективность процесса гидрооблагораживания масла – продукта быстрого пиролиза лигноцеллюлозный биомассы – в присутствии катализатора Co-Mo/Al2O3. Определены относительные вклады скоростей деметилирования, изомеризации, трансалкилирования, гидрирования и ГДО.
Помимо устных докладов, в рамках секции была проведена дискуссия на тему “Возможность использования биомассы для производства топлив и химикатов”. Был обсужден ряд конкретных процессов – с точки зрения их химизма и кинетики, а также с позиций доступности сырья, локальной и глобальной потребностей в конкретных продуктах и прочих технико-экономических факторов. Участники пришли к мнению, что создание научной базы соответствующих технологий является не только оправданным, но и необходимым.
Секция № 2 “Приготовление и характеризация катализаторов” охватывала широкий круг работ, ориентированных как на проведение модельных исследований, так и на решение практических задач гетерогенного катализа.
В первый день работы Конгресса на секции 2 были предоставлены доклады, посвященные применению методов магнитного ядерного резонанса в катализе. И. Иванова (Москва, Россия) представила результаты исследования синтеза цеолитных катализаторов. В докладе был сделал акцент на возможность применения метода ЯМР в режиме in situ. К. Ковтунов (Новосибирск, Россия) рассказал о новом подходе к изучению механизмов гетерогенных каталитических реакций, основанном на применении эффекта индуцированной пара-водородом поляризации ядер. Е. Талзи (Новосибирск, Россия) представил новые результаты исследования одноцентровых каталитических систем для полимеризации этилена.
Второй день был посвящен применению in situ методов для исследования механизмов гетерогенных каталитических реакций. М. Хавекер (Havecker, Берлин, Германия) рассказал о новой станции в центре синхротронного излучения BESSY-II, позволяющей изучать электрохимические процессы методами рентгеновской фотоэлектронной спектроскопии и спектроскопии рентгеновского поглощения. А. Клюшин (Берлин, Германия) представил новые результаты in situ РФЭС исследования реакции окисления СО на поверхности нанесенных золотых наночастиц. В. Каичев (Новосибирск, Россия) рассказал о механизме селективного окисления этанола в ацетальдегид и уксусную кислоту на поверхности монослойных ванадий-титановых катализаторов, построенном на основе результатов in situ исследований методами РФЭС и ИК спектроскопии. Доклад Д. Землянова (Западный Лафайет, США) был посвящен созданию модельных катализаторов методом атомно-слоевого осаждения (ALD – atomic layer deposition) и их исследования в реакции окисления СО.
Третий день был посвящен квантово-химическим исследованиям. Д. Пичугина (Москва, Россия) представила результаты расчетов реакции окисления водорода на поверхности золотых наночастиц. Х.Дж. Венвик (Тронхейм, Норвегия) рассказала о поверхностных явлениях, влияющих на активность Pd-Ag сплава. И.А. Филот (Эйндховен, Нидерланды) посвятил свой доклад механизму селективного гидрирования СО на родии.
Кроме того, были представлены результаты каталитических исследований ряда практически важных реакций. Ю. Гуляева (Новосибирск, Россия) представила результаты исследования селективного (до этилена) гидрирования ацетилена на палладии. Доклад Дж.С. Мартинез-Эспина (Осло, Норвегия) был посвящен изучению процесса метанирования бензола. М. Быкова (Новосибирск, Россия) рассказала о процессах каталитической переработки бионефти на медно-никелевых катализаторах. Ф. Фрустери (F. Frusteri, Мессина, Италия) представил результаты исследования процесса получения биотоплива из глицерина на каталитическом мембранном реакторе.
Программа стендовых докладов секции 2 насчитывала 245 презентаций, представляющих результаты исследования широкого круга каталитических реакций. Наибольшее количество работ было посвящено изучению процессов нефтепереработки, получения водорода из метана, метанола и этанола, окисления СО, синтезу Фишера-Тропша, восстановлению NO. Ряд работ, несомненно заслуживающих особого внимания, был посвящен применению различных in situ методов для исследования механизмов гетерогенных каталитических реакций. Наибольшее количество работ по этой тематике представили ученые из Германии, Австрии и России. Традиционно здесь были представлены исследования, выполненные с помощью методов рентгеновской фотоэлектронной спектроскопии и колебательной спектроскопии.
Следует отметить, что на данном Конгрессе были слабо представлены теоретические работы. Например, из российских работ можно выделить лишь квантовохимические расчеты модельных систем, выполненные в МГУ и в Институте химии и химической технологии СО РАН. Также на удивление мало было представлено работ, посвященных изучению катализа на золоте.
На секции № 3 “Энергетический катализ” были представлены работы от фото- и электрокаталитического преобразования солнечной энергии до технологий создания современных топливных элементов.
Большим разнообразием тематик отличалась программа устных докладов. Как и в программе ключевых лекций, большое внимание было уделено теме синтеза и исследования перспективных фотокатализаторов для энергоэффективных процессов. Исследованию фотокатализаторов выделения водорода были посвящены доклады Е. Козловой (Россия), А. Ремпеля (Россия), Ф. Вейде (Германия), К. Маршал (Франция), К. Санвальд (Франция). О проблемах фотоэлектрохимического и электрохимического разложения воды рассказали М. Мюлер (Германия), Л. Гао (Нидерланды), C. Покрант (Швейцария), Д. Тешнер (Германия), Н. Вейдлер (Германия), К. Нерви (Италия), А. Ощепков (Россия). Перспективному процессу фотокаталитического восстановления углекислого газа были посвящены устные доклады А. Пуга (Испания) и Х. Фариа (Португалия).
Другим широко представленным направлением в работе секции 3 были доклады по паровой конверсии метана, метанола и этанола, представленные Г. Константиновым, В. Садыковым (Россия), С. Ангели (Греция), Р. Ватанабе (Япония), Р. Мафесанти (Италия), П. Кастано (Испания), A. Лыткиной (Россия), К. Лукарелли (Италия) и Р. Талингер (Австрия). Большой интерес вызвали доклады П. Снытникова (Россия) и Г. Ланди (Италия), посвященные каталитическим методам очистки синтез-газа от угарного газа для дальнейшего использования в топливных элементах.
Большое внимание привлекли доклады по новым каталитическим технологиям получения водорода. Работы по синтезу катализаторов получения водорода из муравьиной кислоты представили Д. Булушев (Россия) и Э. Озенсой (Турция); В. Симагина (Россия) представила новые результаты по выделению водорода из боргидридов натрия; Т. Белл (Англия) рассказала о новых катализаторах низкотемпературного разложения аммиака на водород и азот.
В ходе Конгресса были представлены работы по синтезу новых углеродсодержащих топлив и их компонентов. Тремя докладчиками – Э. Фрей (Германия), Л. Петров (Саудовская Аравия), Й. Кирхнер (Германия) – были рассмотрены вопросы каталитического синтеза метанола из углекислого газа. Доклады по синтезу Фишера-Тропша были представлены В. Чен (Нидерланды), К. Ченг (Китай) и Дж. Джайкоди Каруппия (Корея). Й. Антон (Германия) представил работу по каталитическому синтезу высших спиртов; темой доклада Ф. Фрустери (Италия) был синтез биотоплив путем этерификации глицерина; Х. Хуанг (Нидерланды) рассказал о деполимеризации лигнина с образованием ароматических углеводородов. Дж. Мартинез-Эспин (Норвегия) сделал доклад о каталитическом метилировании бензола. Большой интерес вызвал доклад Дж. Хе (Германия) по каталитической гидрогенизации диарилов с использованием различных растворителей. О важной технологии каталитического парциального окисления рассказали В. Арутюнов (Россия) и А. Беретта (Италия).
В программе стендовых докладов секции 3 было заявлено более 120 постеров. Если в программе устных докладов преобладали работы по фото- и электрокатализу, то среди стендовых самым широко представленным направлением являлось получение водорода для топливных элементов путем паровой и углекислотной конверсии метана, а также паровой конверсии спиртов. Было сделано довольно большое количество докладов по каталитическому синтезу и облагораживанию биотоплив, а также по синтезу Фишеру-Тропша. Интерес вызвали стендовые доклады по современным методам сероочистки углеводородных топлив. Большинство стендовых докладов было представлено молодыми учеными из разных стран, что способствовало интересной и продуктивной дискуссии, а молодым ученым из России позволило получить неоценимую практику обсуждения научной работы на английском языке. Большое внимание участников привлекли постеры аспирантов Института катализа СО РАН (Россия) А.А. Печенкина по теме “Сравнительное изучение катализаторов CuO-CeO2 и CuO-ZnO, нанесенных на оксид алюминия для парового риформинга диметоксиметана” и И.В. Шаманаева по теме “Гидродеоксигенация алифатических эфиров на катализаторах на основе фосфида никеля”.
Кроме устных и стендовых докладов, по презентациям секции 3 проводился дискуссионный симпозиум “Фотокатализ: разложение воды, восстановление СО2 и окислительные процессы”. В программу Симпозиума вошли наиболее интересные стендовые доклады в области фотокатализа, представленные на Конгрессе. В программе двухчасового Симпозиума было сделано пять докладов, что позволило участникам всесторонне обсудить каждое выступление. Дж. Джиао (Китай) и К. Штриглер (Германия) рассказали о новых результатах в области фотокаталитического восстановления углекислого газа на композитных фотокатализаторах. Профессор Э. Озенсой (Турция) сделал доклад о синтезе новых биомиметических структур – букиболов – для реакций фотокаталитического окисления. Сотрудники Института катализа (Россия) Д.С. Селищев и П.А Колинько рассказали о новых подходах в области создания комплексных систем фотокаталитической очистки воздуха. После докладов состоялась активная дискуссия, основным вопросом которой были критерии качества для статей в области фотокатализа. Участники Симпозиума пришли к выводу, что основной проблемой является унификация фотокаталитической активности, в том числе корректное сравнение активности новых материалов с активностью известных коммерческих образцов.
Секция № 4 “Катализ и химические соединения” была посвящена каталитическим реакциям селективного синтеза сложных органических соединений.
Большим разнообразием тематик отличалась программа устных докладов. Так, целый ряд устных докладов (N. Perret, В.А. Лихолобов, F. Cárdenas-Lizana, E. Nowicka, O. Beswick, N. D’Allessandro) был посвящен различным катализаторам и процессам селективной каталитической гидрогенизации органических соединений. Рассматривались как процессы восстановления водородом, так и возможности реализации сопряженного процесса (F. Cárdenas-Lizana), в который вовлекаются молекулы органического восстановителя (источника двух атомов водорода) и окислителя (принимающего два атома водорода).
Несколько докладчиков (К.Ю. Колтунов, К.П. Брыляков, Z.G. Zhang, F. Cavani, V. Nese, A. Padovani, Т.П. Отрощенко, О.Т. Касайкина, A. Berenguer-Murcia, B. Schaller) представили результаты исследования различных реакций селективного (в том числе энантиоселективного) каталитического окисления органических соединений с образованием связей С-О, С-С, гетероатом-О и т.д. К.П. Брыляковым был представлен новый, экологически безопасный способ стереоселективного каталитического синтеза эффективного противоязвенного препарата (S)-омепразола. Ряд работ (E. I. Ruppel, V. Hulea) был посвящен различным процессам олиго- и полимеризации олефинов на гомогенных и гетерогенных катализаторах.
Получению различных функционализированных органических соединений были посвящены доклады А.А. Трифонова, Л.В. Парфеновой, М.Н. Хризанфорова, М. Розе, О.Н. Мартьянова, Д.Б. Расмуссена, А.О. Терентьева, И. Жу.
В соответствии с направленностью всего Конгресса (“сбалансированное использование ископаемых и возобновляемых ресурсов”), в секции 4 большое внимание уделялось каталитическим превращениям, в которые вовлекаются различные виды возобновляемого химического сырья растительного происхождения. Так, переработке и селективным превращениям разнообразных моно- и полисахаридов были посвящены доклады В.Н.П. Ван-Дер-Граафa, Е.А. Пидько, С. Тольборга, И. Делидович, А. Падовани, С.М. Коман, Е. Жирара, И. Ванг, Т. Йокой, О.Т. Касайкиной. В работе А. Ауроу изучались каталитические процессы синтеза 1-бутанола и 1-пропанола из смеси метанола и этанола на основных катализаторах. Интересную работу представила М. Ботавина, предложившая катализаторы для синтеза органических карбонатов из С1-С4 спиртов и углекислого газа, который в большом количестве присутствует в составе атмосферы Земли.
В то же время, заметный удельный вес имели доклады, посвященные синтезу сложных органических молекул из простых исходных соединений, представляющих собой либо природные углеводороды (главным образом метан), либо продукты первичной переработки природных углеродсодержащих ископаемых (синтез-газ). Авторами рассмотрен ряд новых каталитических процессов прямого получения метанола из метана (B. Schaller), сухого риформинга метана (H. Atia), синтеза углеводородов из метанола на цеолитах (J. K. Erickson), прямого синтеза диметилового эфира из синтез-газа (H. J. Venvik), получения бензола напрямую из метана в жестких условиях (Z. G. Zhang).
Программа стендовых докладов секции 4 насчитывала 155 презентаций, посвященных различным актуальным проблемам современного катализа. Затрагивались как актуальные вопросы селективных каталитических превращений (окисления, восстановления, в том числе селективного гидрирования, дегидрирования, ароматизации, гидродеароматизации, алкилирования, ацетилирования, синтеза амидов, карбонилирования, перегруппировок, изомеризации, активации С-Н связей, C-C сочетания, диенового синтеза, этерификации, биохимических процессов и др.), так и различных реакций получения углеводородов (координационной и метатезисной полимеризации, сополимеризации и олигомеризации олефинов, синтеза Фишера-Тропша, прямой конверсии метана в ароматические углеводороды, крекинга и др.). Можно отметить также несколько работ, посвященных использованию энергии электромагнитного излучения в каталитических процессах (Т.М. Федорова, E. García-López, M.S. Hamdy, A.D. Erdogan, Г.А. Зенковец).
В большом количестве докладов (V.I. Parvulescu, S. Chornaja, W.P. Deng, D.M. Mounguengui, E. Diaz, А.А. Степачева, E. García-López, Н.В. Громов, Y.M. Isa, Б.Н. Кузнецов, A.A, Lemonidou, K.S. Lin, E. Lombardi, E. Girard, Y. Zhu, A.E. Modvig, C.M. Opris, S. Ordonez, G. Garbarino, S.M. Coman, J.M. Thybaut, E. Redina, V.L. Sushkevich, L.M. Thomas, J. Velasquez Ochoa, T. Wang, K.Y. Chul, G.G. Sub, Г.А. Коваленко) был сделан акцент на разработку каталитических методов превращения возобновляемого сырья (глицерина, 5-гидроксиметилфурфураля, спиртов, целлюлозы, древесины, глюкозы, лигнина, других полисахаридов, диоксида углерода) в ценные химические продукты. Это является наглядным подтверждением актуальности направленности Конгресса на переработку возобновляемых ресурсов.
Рассматривались различные аспекты приготовления катализаторов с заданными свойствами, практической реализации каталитических превращений, оптимизации реакционных условий, использовании нетрадиционных растворителей (ионных жидкостей, сверхкритического CO2), характеризации катализаторов физико-химическими методами, математического моделирования каталитических процессов, изучения механизмов каталитического действия физическими и теоретическими (расчетными) методами.
Доклады секции № 5 “Катализ и защита окружающей среды” отражали последние тенденции в области катализа в защите окружающей среды, а именно, использование катализа как для удаления вредных веществ из газовых выбросов или жидких стоков, так и для трансформации парниковых газов (метан, диоксид углерода) и отходов переработки биомассы в синтез-газ, водород и синтетические топлива, а также ценные химические продукты.
В работах D. Cani (Бельгия), Д. Козлова (Россия), J. Hirayama (Япония) были рассмотрены проблемы использования фотокатализа для удаления органических и неорганических примесей из газов и жидких растворов. Показана высокая эффективность фотокатализаторов на основе уранила, нанесенного на оксиды титана, алюминия и кремния.
В работе Е. Пархомчук (Россия) представлены результаты по дизайну катион-замещенных цеолитов с иерархической пористой структурой и их каталитические свойства в реакциях глубокого окисления органических примесей перекисью водорода в жидкой фазе.
В докладах G. Garbarino (Италия), L. Falbo (Италия), G. Bonura (Италия), З. Букиной (Россия), М. Бухтияровой (Россия) рассмотрены проблемы подбора эффективных катализаторов для процессов гидрирования оксидов углерода с получением различных синтетических топлив и влияния состава реакционной смеси и различных примесей на параметры процессов и стабильность катализаторов. Так, G. Garbarino показано, что промышленный катализатор Ru/Al2O3 является активным, селективным и стабильным катализатором метанирования СО2, что открывает перспективы его практического применения в данном процессе.
Большое внимание было также уделено процессам трансформации СО2 в синтез-газ в реакциях углекислотной или смешанной конверсии природного газа (P.M. Mortensen, Дания; M. Gaillard, Франция). Показано, что можно обеспечить устойчивость к зауглероживанию промышленных нанесенных никелевых катализаторов углекислотной конверсии метана путем добавления диоксида серы к реакционной смеси.
Проблемы дизайна структурированных катализаторов для очистки отходящих газов от органических соединений были рассмотрены в докладах С. Яшник (Россия), А. Сукнева (Россия), D. Lopez-Gonzalez (Франция) и др. Показано (А. Сукнев), что нанесение платины на стекловолокнистый носитель позволяет создать высокоэффективный катализатор полного окисления органических примесей в воздухе.
Проблемы кинетики и механизма реакций полного и селективного окисления углеводородов были рассмотрены в докладах А. Худорожкова (Россия), H. Stotz (Германия). Показано, что активность и селективность нанесенного на окись алюминия палладия в реакциях полного/селективного окисления пропана определяются соотношением катионных и металлических состояний палладия. Для нанесенного палладия процесс селективного окисления метана в синтез-газ включает в себя маршрут полного окисления в зоне реактора, содержащей кислород, с последующей реализацией процессов паровой и углекислотной конверсии.
Был представлен ряд докладов, посвященных использованию СО2 в процессах окислительного дегидрирования алканов в алкены (E. Nowicka, Великобритания) и взаимодействия с эпоксидами с образованием циклических карбонатов в ионных жидкостях (C. Aprile, Бельгия).
Рассмотрены проблемы каталитической трансформации различных природных соединений – компонентов биомассы (глицерин, спирты, муравьиная кислота и пр.) в ценные химические продукты, водород и биотоплива (Ю. Демидова (Россия), A. Lemonidou (Греция), M. Zacharska (Ирландия), М. Цодиков (Россия)).
Были детально рассмотрены проблемы кинетики и механизма реакций селективного восстановления оксидов азота в избытке кислорода с использованием в качестве восстановителя аммиака/мочевины (W. Grünert (Германия), P. Moses (Дания), I. Nova (Италия), J.M. Garcia-Vargas (Франция), P. Granger (Франция), M. Sridhar (Швейцария), Z. Say (Турция)) в основном на катион-замещенных цеолитах разного типа, а также на оксидных катализаторах (CeVO4, Au/TiO2, BaO/ZrO2/TiO2/Al2O3). Работы по схожим тематикам обсуждались в докладах E. Tronconi (Италия), А. Шишкина (Швеция), J.R. Thøgersen (Дания). В докладе S. Alcove (Великобритания) было рассмотрено влияние примесей ртути и HCl в дымовых газах на процесс селективного восстановления оксидов азота на оксидном катализаторе V2O5/MoO3/TiO2.
В ряде работ были представлены результаты исследований процессов комплексной очистки отходящих газов автотранспорта (в том числе окисление сажи) на катализаторах, содержащих смешанные оксиды церия-циркония (A. García-García (Испания), А. Порсин (Россия), J. Zheng (Китай)), Ag/YSZ (A. Serve, Франция), оксиды железа (S. Kureti, Германия), оксиды переходных металлов, промотированные калием (A. Kotarba, Польша), перовскиты (S. Heikens (Германия), H. Arandiyan (Австралия), L. Tang (Китай)).
Разложение закиси азота на оксидных катализаторах (шпинели Mn3O4, Fe3O4, Co3O4, K-CuxCo3-xO4, CuO-CeO2) было рассмотрено в докладах Z. Sojka (Польша), T. Franken (Германия), E. Papista (Греция), M. Zabilskiy (Словения). Было показано, что данные оксидные системы обладают высокой активностью и достаточной для практического использования стабильностью. Проанализированы атомно-молекулярные характеристики, определяющие активность таких систем.
В докладе C. Bradu (Румыния) показано, что ионообменные смолы, промотированные Pd-Cu, обеспечивают высокую активность в реакциях восстановления нитратов в водных средах и обладают необходимой стабильностью.
***
Одной из отличительных особенностей Конгресса EuropaCat-2015 стал акцент на использование возобновляемого сырья для получения топлива и ценных органических продуктов, в том числе в рамках хирального/энантиоселективного катализа/биокатализа. С этим в значительной степени связано и большое количество работ, рассматривающих проблемы фотокатализа.
Следует отметить очень широкую представленность в программе Конгресса (и во второй секции, и в других) работ, связанных с детальным изучением катализаторов комплексами современных структурных и спектральных методов (включая уникальные особенности европейских и российских центров синхротронного излучения, нейтронных источников и т.п.) и механизма каталитических реакций с использованием методов in situ и operando, а также с применением квантово-химических расчетов. Это отражает современные тенденции установления атомно-молекулярных основ каталитического действия, необходимых для целенаправленного дизайна катализаторов, в то время как комбинаторный подход используется в основном для первоначального скрининга катализаторов.
В области каталитических методов защиты окружающей среды начинают быстро развиваться подходы, связанные с трансформацией парниковых газов (диоксида углерода, метана) в топливо и ценные химические продукты. Среди наиболее интересных работ Конгресса в данном направлении можно отметить работу профессора К. Априле (Намюр, Бельгия) по взаимодействию СО2 с эпоксидами в ионных жидкостях на мезопористом SiO2 с образованием циклических карбонатов.
Существенное внимание было уделено различным проблемам синтеза новых перспективных наноматериалов на основе фуллеренов, углеродных нанотрубок, полимеров и т.д., а также структурированных катализаторов и каталитических мембран, что также находится в русле современных тенденций катализа.
Программа Конгресса включала также презентации XI Международного симпозиума по достижениям в селективном окислении, что позволило осветить проблемы целенаправленного дизайна катализаторов селективного окисления, специфики кинетики и механизмов таких реакций и подходов к оптимизации параметров процессов и конструкций реакторов на основе математического моделирования для управления селективностью по целевым продуктам.
Впервые в рамках конгресса EuropaCat-XII прошел симпозиум “Образование в области катализа”. На Симпозиуме обсуждались вопросы учебных программ в области гетерогенного катализа, как для вступительных лекционных курсов, так и для курсов, разработанных для более подготовленных слушателей (аспиранты 2 – 4 годов обучения). Было проведено сопоставление подходов, используемых в различных европейских странах. Важной инициативой, предложенной российской стороной, стал обмен лекционными материалами между учеными разных стран, который предполагается организовать на уровне национальных каталитических и химических обществ. В рамках Симпозиума детально обсуждался вопрос подготовки и проведения практических работ в области гетерогенного катализа, которые необходимы учащимся не только для закрепления знаний, полученных при прослушивании теоретических курсов, но и для получения практических навыков в таких областях как приготовление катализаторов и их характеризация различными физико-химическими методами. По общему мнению участников, обмен мнениями по поводу возможной организации и совершенствования методов преподавания гетерогенного катализа был чрезвычайно полезен и интересен для делегатов всех стран-участниц Симпозиума.
Материалы избранных докладов XII Европейского конгресса по катализу будут опубликованы в специальных выпусках международных рецензируемых журналов Catalysis Today (Elsevier) и Topics in Catalysis (Springer).
Следующий, XIII Европейский конгресс по катализу (EuropaCat-2017) состоится во Флоренции (Италия) 27-31 августа 2017 года.
Материал подготовили
В.И. Бухтияров, В.Н. Пармон, В.В. Каичев, К.П. Брыляков, Е.А. Козлова,
Л.Я. Старцева (ИК СО РАН, Новосибирск),
А.Ю. Стахеев, О.В.
Турова, И.С. Машковский (ИОХ РАН, Москва),
М.Ю. Синев (ИХФ РАН,
Москва)
в рамках XII Европейского конгресса по катализу
1-3 сентября 2015 г. (Казань, Россия)
Симпозиумы по селективному окислению, проведенные ранее в Италии (1999 г.), Австрии (2003 г.), Финляндии (2007 г.), Испании (2009 г.) и Шотландии (2011 г.), позволили установить и развить контакты между специалистами ведущих исследовательских центров мира в области селективного окисления, определить наиболее перспективные направления в области разработки высокоэффективных катализаторов селективного окисления различных видов сырья, в том числе возобновляемого, в ценные химические продукты, изучения кинетики и механизма реакций, что позволило заложить фундаментальные основы соответствующих промышленных процессов. Симпозиумы способствовали координации и объединению усилий специалистов для участия в международных проектах, финансируемых в рамках программ Европейской комиссии, и других с участием российских ученых. Настоящий Симпозиум продолжает традиции прошедших мероприятий по направлению селективного окисления.
В состав 90 участников Симпозиума вошли российские и зарубежные специалисты, а также студенты и аспиранты. Программа Симпозиума включала две ключевых лекции (30 мин), 24 устных доклада, 12 кратких устных докладов на флэш-сессии и 27 стендовых докладов.
Ключевая лекция д.х.н. О.А. Холдеевой была посвящена применению металл-органических каркасов, в частности семейства MIL, в реакциях жидкофазного селективного окисления. Подробно обсуждались вопросы влияния природы переходного металла, роль диффузии и адсорбции на селективность реакций парциального окисления углеводородов с использованием таких мягких окислителей, как перекись водорода и кислород воздуха; на основе исследования стабильности катализаторов предложены рекомендации по выбору реакционных условий, а также примеры разработанных высокоактивных и регенерируемых катализаторов.
Повышенный интерес вызвала ключевая лекция профессора Гюнтера Колба (Gunter Kolb) о возможностях применения микрореакторов в реакциях селективного и неполного окисления для производства ценных химических продуктов, таких как этиленоксид, бензальдегид, бензойная кислота, перекись водорода. Поднимались вопросы о масштабировании процессов, проблемах массо- и теплопереноса, безопасности из-за высокоэкзотермического характера реакций окисления. Обсуждались подходы к решению проблем, возникающих при подготовке водородсодержащего газа для топливных элементов. Продемонстрирована созданная коллективом автора доклада установка очистки синтез-газа от монооксида углерода с использованием теплопроводящих носителей и рекуперацией энергии процесса окисления.
Устные доклады были посвящены изучению широкого круга реакций и катализаторов для переработки как ископаемого сырья, так и возобновляемых ресурсов. В целом тематика устных докладов совпадала с проблематикой, озвученной авторами ключевых лекций. Отмечая высокий уровень представленных исследовательских работ, важно упомянуть, что 9 из 24 устных докладов были сделаны молодыми специалистами. Окислительные превращения спиртов – одно из перспективных направлений, которому уделялось повышенное внимание участников Симпозиума. Доктор Хосе Лопес Нието (Dr. Lopez Nieto Jose) открыл работу секции устных докладов презентацией, посвященной превращениям глицерина и метанола в аэробных условиях в присутствии промотированных вольфрамовых катализаторов. В работе показано, что изоморфное замещение катионов вольфрама на катионы ниобия, титана или ванадия изменяет окислительно-восстановительные и кислотно-основные свойства катализаторов и открывает возможности для увеличения их активности. В докладе Лауры Прати (Prati Laura) рассматривались реакции окисления бензилового спирта до бензальдегида и бензойной кислоты в присутствии наноразмерных частиц золота и палладия, нанесенных на пористый оксид титана; роль золота заключалась в подавлении необратимой адсорбции, что приводило к увеличению стабильности и селективности. К.х.н. Михаил Симонов показал в своем докладе, как методами изотопно-кинетических релаксаций и микрокалориметрии можно установить основные маршруты превращений этанола в синтез-газ на промотированном платиной оксидном катализаторе с высокой подвижностью решеточного кислорода. Доктор Висенте Кортес Корберан (Dr. Cortes Corberan Vicente) представил результаты исследования реакции окисления тетрадеканола в присутствии наноразмерного золота, нанесенного на оксид церия-алюминия. Путем варьирования условий, таких как температура, соотношение катализатора к субстрату и время реакции, возможно увеличить выход альдегида до 30% с селективностью до 90%. Селективное окисление октанола на промотированных золотых и серебряных катализаторах – тема доклада д.х.н. Алексея Пестрякова, где продемонстрировано, что активными центрами являются частично заряженные кластеры металлов, а дополнительное увеличение активности катализатора происходит за счет модифицирования носителя на основе оксида титана. Как показал Евгений Хаскин в своем докладе, значительный прогресс достигнут в гомогенном окислении спиртов без использования окислителя. В качестве источника кислорода может быть использована вода, и путем подбора подходящего реагента реакция окисления может быть остановлена на стадии образования ценного альдегида или кетона. Получение адипиновой кислоты – прекурсора для промышленного синтеза нейлона – тема доклада Стефании Солми (Solmi Stefania). В презентации автор описала гетерогенные и гомогенные катализаторы окисления циклогександиола в целевой продукт и представила общую схему превращений реагентов и интермедиатов, объясняющую видимые кинетические закономерности.
Два доклада были посвящены получению эпоксидов из соответствующих алкенов. Так, новый класс катализаторов эпоксидирования на основе не содержащих металлов модифицированных углеродных материалов был предложен профессором Паоло Пескармона (Prof. Pescarmona Paolo). Немаловажно, что катализаторы данного типа перспективны с точки зрения так называемой “зеленой химии”. Теоретическую работу по изучению процесса получения пропиленоксида из пропилена на золотосодержащих катализаторах представила доктор Людмила Москалева. Автор полагает, что высокая селективность эпоксидирования в присутствии воды и водорода связана с образованием пероксидных группировок in situ.
Низкотемпературное окисление на серебросодержащих катализаторах – тема устного доклада к.х.н. Григория Мамонтова, где автор показал, что сильное взаимодействие металл-носитель между серебром и оксидом церия играет ключевую роль в окислении органических веществ, в то время как слабое взаимодействие между наночастицами серебра и оксидом кремния является причиной низкотемпературной активности в реакции окисления монооксида углерода.
Из сообщения доктора Хосе Одриозола Гордона (Dr. Odriozola Gordón José) участники Симпозиума узнали, что ионные жидкости могут использоваться не только в качестве растворителей, но и как часть гибридного катализатора превращений глюкозы в глюконовую кислоту.
Можно отметить работу доктора Венжи Шеня (Dr. Shen Wenjie), в которой показана корреляция между каталитическими свойствами и морфологией контактирующих с реакционной средой оксидов металлов.
К.х.н. Роман Оттенбахер в своем докладе пролил свет на механизм реакций селективного окисления с использованием биомиметических гомогенных катализаторов на основе аминопиридиновых комплексов марганца и показал, что активным центром в данном случае является MnV-оксо группа.
Переработка лигноцеллюлозы, а точнее, продуктов ее утилизации, таких как фурфураль и его производные, является перспективным процессом использования отходов для синтеза ценных соединений. Доклад доктора Сильинды Ператонер (Dr. Perathoner Siglinda) был посвящен изучению окислительной этерификации фурфураля метанолом в присутствии золотосодержащих катализаторов на основе оксидов церия и циркония; показано, что данный процесс может открыть новые перспективы в тонком органическом синтезе. Профессор Себастьен Пол (Prof. Paul Sebastien) представил доклад о селективном окислении 5-гидроксиметилфурфураля в 2,5-диформилфуран на гетерогенном оксофосфате ванадия с высоким выходом и селективностью. Схожие результаты продемонстрировала Михаэла Флореа (Ph.D. Florea Mihaela), с тем отличием, что катализаторы представляли собой смешанные оксиды марганца-меди.
Часть работ была связана с исследованием таких реакций превращения метана, как окислительная конденсация и селективное окисление. Так, работа, представленная доктором Клодом Миродатосом (Dr. Mirodatos Claude), была посвящена изучению влияния соотношения объема к поверхности и позволяет численно обосновать и прогнозировать эффективность действия катализатора, а также задает направление в проектировании и оптимизации реакторов. Д.х.н. Михаил Синев в своем докладе осветил такие аспекты, как реакционноспособность решеточного кислорода, роль компонентов в смешанной оксидной системе, а также взаимоотношения между составом, структурой, доступностью кислорода и каталитической активностью. К.х.н. Дмитрий Иванов продемонстрировал, что в условиях окислительного связывания метана происходит перестройка поверхностного состава и микроструктуры титанатов стронция с появлением фаз смешанных оксидов на поверхности исходных титанатов, что сильно влияет на каталитические свойства и на процесс гетерообмена кислорода между поверхностью и объемом. Явление кинетического гистерезиса в ходе парциального окисления метана, обнаруженное к.х.н. Ильей Пахаруковым, заключается в том, что активность и селективность платинового катализатора существенным образом определяются “историей” достижения стационарного состояния. Кинетический гистерезис позволяет осуществлять непосредственный контроль над активностью и селективностью катализатора, например, на платине можно увеличить конверсию с 10 до 90% без изменения состава катализатора или температуры. К.х.н. Евгений Староконь в своем докладе сравнил квазикаталитический и каталитический режимы реакции окисления метана в метанол с участием альфа-кислорода, что позволило автору идентифицировать возможные интермедиаты и представить механистические схемы превращений, происходящих в обоих режимах.
Презентация Ишикавы Сатоши (Ishikawa Satoshi I.) была посвящена окислительной конверсии этана в этилен; автор убедительно показал взаимосвязь кристаллической структуры, микропористости и каталитической активности оксида ванадия-молибдена в данной реакции. Доктор Иоан-Цезар Марку (Dr. Marcu Ioan-Cezar) рассказал о корреляциях между каталитическими свойствами и электропроводностью допированных оксидов никеля и представил прямое доказательство того, что гетерогенный окислительно-восстановительный процесс окисления этана в этилен проходит с участием поверхностных ионов кислорода решетки.
Д.х.н. Оксана Таран представила интересный доклад о селективном окислении полисахаридов до муравьиной кислоты на гетерополикислотных катализаторах. Был сделан важный вывод о том, что в присутствии фосфор-молибден-ванадиевой гетерополикислоты окислительный гидролиз проходит по механизму, схожему с механизмом периодатного окисления.
Короткие устные доклады на флэш-сессии были непосредственно связаны с тематикой Симпозиума и посвящены таким направлениям, как окислительные превращения метана в метиловый спирт, этилен и синтез-газ, окисление пропилена до акриловой кислоты и пропиленоксида, окисление одно- и многоатомных спиртов и сахаров, окисление монооксида углерода, а также фотокаталитические процессы окисления сложных органических веществ.
Тематика стендовых докладов, помимо научных направлений Симпозиума, включала и другие направления: приготовление и использование магнитных катализаторов, окисление этилового, пропилового, бензилового спиртов в альдегиды и кетоны, окислительное дегидрирование этана, пропана, метилбензола в соответствующие алкены, селективное окисление циклогексана и бензола до спиртов, а также некоторые направления тонкого органического синтеза: окислительное фосфорилирование гетероарилазолов и получение 2-метил-1,4-нафтохинона.
На церемонии закрытия была отмечено, что работы по окислительному катализу были актуальными и в большинстве своем представлены на высоком уровне: продемонстрированы последние достижения в развитии методов проведения и исследования реакций окисления, исследования в области направленного синтеза катализаторов окисления; были показаны новые результаты в области окислительного катализа с использованием возобновляемого сырья. Особо была подчеркнута активность молодых специалистов, принявших участие в Симпозиуме в качестве докладчиков и слушателей, что свидетельствует о стабильном интересе молодежи к высокоуровневым научным исследованиям и преемственности поколений научных школ.
Материалы XI Международного симпозиума по селективному окислению будут опубликованы в специальном выпуске Catalysis Today (Elsevier) и Catalysis for Sustainable Energy.
Следующий, XII Международный симпозиум “Селективное окисление как ключ к повышению ценности новых ресурсов” состоится в 2017 году в рамках Мирового конгресса по окислительному катализу в Кракове (Польша).
Материал подготовили
М.Н. Симонов, В.А. Садыков, И.В. Красникова
(ИК СО РАН, Новосибирск)
в рамках XII Европейского конгресса по катализу
3 сентября 2015 г. (Казань, Россия)
На прошедшем в 2014 году Российском конгрессе по катализу “Роскатализ” (Самара, 2-5 октября 2014 г.) был успешно проведен симпозиум “Образование и катализ”, посвященный анализу проблемы преподавания каталитической науки в высшей школе. В связи со значительным успехом этого мероприятия и интересом к нему со стороны ученых, преподавателей и производственников, работающих в области катализа, данная тематика была предложена для обсуждения на XII Европейском конгрессе по катализу. После тщательного рассмотрения и не без колебаний Европейская ассоциация каталитических обществ (EFCATS) – основной организатор XII Европейского конгресса по катализу – приняла решение о включении симпозиума “Образование в области катализа” (“Education in the field of catalysis”) в программу Конгресса.
В Симпозиуме приняли участие представители многих стран, общее количество участников превысило 150 человек. Наибольшее количество докладов сделали представители России, Германии, Франции, Италии, Турции. Участвовали также ученые и педагоги из Швеции, Нидерландов, США, Австралии, Греции, Китая, Кореи, Индии, Сингапура, Дании, Финляндии и других стран.
На исключительную важность каталитической тематики, обсуждаемой на Конгрессе, и тематики Симпозиума указывают выступления Президента Республики Татарстан Р.Н. Минниханова на открытии Конгресса и Министра образования и науки РФ Д.В. Ливанова, который особо подчеркнул своевременность и насущную необходимость проведения симпозиума “Образование в области катализа” в рамках Конгресса Европакат-XII.
Наряду с обсуждением передовых образовательных методик, на Симпозиуме молодые ученые и педагоги получили возможность представить свои последние достижения в образовательной области, а также научные разработки в области теоретических основ катализа и использования физико-химических методов для изучения механизмов каталитических процессов. В программе были предложены следующие темы для обсуждения:
В программу Симпозиума вошла пленарная секция. Кроме пленарной секции, состоялись два заседания с устными докладами, а также масштабная стендовая секция, на которой было представлено 126 докладов.
Председателями пленарной секции были председатель оргкомитета, декан химического факультета МГУ имени М.В. Ломоносова, академик РАН В.В. Лунин и профессор университета Або/Турку (Финляндия) Д.Ю. Мурзин. На секции с докладами выступили представители мировой каталитической науки:
В.В. Лунин (МГУ имени М.В. Ломоносова, Россия) “Образование в области катализа: проблемы и перспективы”,
Д.Ю. Мурзин (Университет Або/Турку, Финляндия) “Преподавание гетерогенного катализа химикам-технологам”,
М. Мюлер (Университет Рура, Бохум, Германия) “Обучение катализу в Германии”,
Ф. Кавани (Университет Болоньи, Италия) “Катализ для химической промышленности, преподаваемый химикам-технологам: примеры из преподавания в Италии”,
М. Скоглунд (Технологический университет Чалмерс, Швеция) “Занятия по катализу в Технологическом университете Чалмерс в Швеции”,
Д. Белтрамини (Университет Квинсленда, Австралия) “Преподавание катализа и технологий проведения реакций в австралийских реалиях”,
В.И. Бухтияров (Новосибирский государственный университет, Россия) “Исследовательская работа как необходимая часть образования в области катализа”,
Х. Харлампиди, Г. Елиманова (Казанский национальный исследовательский технологический университет, Россия) “От лабораторных исследований к промышленным реакторам”,
Э.Д.М. Хенсен (Эйндховенский технологический университет, Нидерланды) “Образование в области катализа при обучении магистров и аспирантов в Нидерландах”,
С.В. Джонс (Технологический институт Джорджии, США) “Каталитическое образование в США”.
Анализ данных, приведенных в докладах, выявил интересные тенденции. Как показано в докладах М. Мюлера, Д. Мурзина и С.В. Джонса, в Германии, Финляндии, других европейских странах и США практически отсутствует координация между университетами в отношении количества, структуры и состава курсов в области катализа. Преподаватели могут даже не знать о том, какие курсы и в каком количестве читаются в других университетах по сравнению с местом их работы. С другой стороны, студенты во многих случаях имеют возможность выяснить, какие курсы читаются в других университетах страны, и пройти соответствующее обучение в виде краткосрочных курсов или долговременных визитов. Такая возможность реализована каталитическими объединениями, организованными университетами Германии (всего 6 объединений, причем они включают не все имеющиеся в этой стране университеты). Кроме того, такая возможность предоставляется студентам соседних стран (так, студенты Австрии и Швейцарии могут пройти соответствующие курсы в Германии). По мнению выступавших, была бы полезна большая степень координации.
В отличие от России, в европейских университетах состав и количество преподаваемых курсов по катализу практически целиком определяются профессорами, работающими в данном университете, в индивидуальном порядке. При этом, в зависимости от состава и направлений предполагаемого трудоустройства студентов, в каждом году состав и структура преподаваемых курсов могут в значительной степени варьироваться, как это показано в докладе Д. Мурзина. В докладе С.В. Джонса также подчеркнута существующая в США децентрализация обучения катализу, отсутствие стандартного набора учебников и содержания курсов, рекомендованных для всех университетов, и относительная индивидуальность обучения в области катализа. Это проявляется как в самостоятельном выборе университетами тематик и содержания курсов по катализу, так и в адаптации обучения под потребности конкретного студента или аспиранта. Существующие в США региональные каталитические объединения специализируются на разных разделах катализа, поэтому значительная роль в объединении и координации усилий этих объединений принадлежит одному из наиболее престижных в отрасли журналу ACS Catalysis, главным редактором которого является докладчик. Участники Симпозиума предложили С.В. Джонсу рассмотреть возможность публикации специального выпуска журнала, посвященного образовательным проблемам. Докладчик отметил, что образовательная тематика поддерживается журналом, и обещал рассмотреть вопрос на редакционной коллегии.
На участников Симпозиума произвели большое впечатление данные о количестве и составе специальных курсов по катализу, читаемых в Новосибирском государственном университете, которые привел в своем докладе В.И. Бухтияров. Тринадцать курсов по катализу, среди которых имеются как общие курсы, например, “Катализ” (В.И. Бухтияров), “Научные основы приготовления катализаторов” (П.А. Симонов), “Молекулярный дизайн катализаторов” (В.Л. Кузнецов), так и уникальные спецкурсы, например, “Термодинамика действующих катализаторов” (В.Н. Пармон), “Магнитная радиоспектроскопия в катализе” (Е.П. Талзи). Кроме того, широко представлены курсы лекций, посвященные использованию в катализе расчетных и современных физико-химических инструментальных методов, без чего невозможно представить определение механизмов катализа и состава активных центров катализаторов. Из аудитории был задан вопрос, все ли перечисленные курсы обязательны для изучения, или же они являются курсами по выбору студентов; однако, как следует из ответа В.И. Бухтиярова, все они обязательны для изучения в НГУ. Сопоставимые по тематике и охвату проблем специальные курсы предлагаются для изучения в МГУ имени М.В. Ломоносова, как подчеркивалось в сообщении декана химического факультета МГУ В.В. Лунина. С этого года химический факультет переходит с пятилетнего на шестилетнее обучение специалистов, и в учебный план дополнительного года включен, например, специальный курс “Избранные главы физической химии”, в рамках которого читается спецкурс по катализу (Е.С. Локтева, Е.В. Голубина) для дипломников кафедры физической химии. Кроме того, на химическом факультете уже давно ведутся специальные курсы “Механизмы каталитических реакций” (В.В. Лунин, Е.С. Локтева), “Научные основы приготовления катализаторов” (В.В. Лунин, Е.В. Голубина), “Применение физико-химических методов в гетерогенном катализе” (каждая лекция читается специалистом по определенному методу), “Нанокатализ” (Б.В. Романовский), а также лекции по приготовлению цеолитных катализаторов (И.И. Иванова, Е. Князева).
Примерно такое же разнообразие каталитических курсов, которое представлено в одном Новосибирском университете, предлагается всеми (но не каждым, а именно всеми) университетами Италии. В своем докладе Ф. Кавани сгруппировал читаемые курсы по типам, основанным на разных принципах: активация молекул, классический металлоорганический подход, инженерный подход, классы каталитических реакций, социально-ориентированный катализ (экологический катализ, катализ в производстве энергии и др.).
Сравнивая учебные планы российских и зарубежных университетов, можно отметить, что в России более формализованные учебные планы, включающие значительно больше готовых и читаемых специальных курсов по катализу, в то время как в европейских университетах курсы более гибкие, их меньше, но они легко и без формальностей адаптируются к потребностям студентов каждого набора. Объясняется такое различие тем, что в России высокая потребность в выпуске химиков, работающих в области катализа, в связи со значительным развитием как химической, так и нефтехимической и нефтеперерабатывающей промышленности, а также полимерной отрасли. Все эти области требуют уверенного владения основами и принципами каталитической науки. В последние годы фармацевтическая промышленность также все больше вовлекает каталитические процессы для приготовления современных лекарств, поскольку новые достижения катализа позволяют проводить сложные органические реакции, такие как кросс-сочетание с участием связей углерод-углерод и углерод-гетероатом, получение чистых оптических изомеров, с использованием меньшего количества стадий и с высокой экономичностью (высокий выход, мягкие условия, высокая селективность).
Кроме того, сравнение данных, приведенных в докладах на Симпозиуме, показывает, что в России более глубоко преподается наука о катализе на ранних стадиях обучения (бакалавры, магистры, специалисты), в то время как за рубежом катализу уделяется серьезное внимание на более продвинутых стадиях обучения (аспирантура).
Практически для всех европейских и российских университетов характерно вовлечение студентов в исследовательскую работу. Различие состоит в том, что в европейских странах студенты, как правило, проводят научную работу в стенах университетов, а в России весьма популярно привлечение к преподаванию, например, руководству курсовыми и дипломными работами, сотрудников исследовательских институтов. Так, в новосибирском Академгородке дипломные работы в области катализа студенты обычно выполняют в Институте катализа им. Г.К. Борескова СО РАН или других институтах СО РАН, и нередко результаты таких работ публикуются в высокорейтинговых международных журналах. Неудивительно, что студенты получают престижные награды и стипендии, например, имени К.И. Замараева, фирм Baker Hughes, Schlumberger, British Petroleum, фонда Потанина и др.
В зарубежных университетах, например, в Италии и Нидерландах, распространяется практика приглашения для преподавания катализа специалистов из соответствующих отраслей промышленности, находящихся на пенсии: они имеют огромный опыт именно в промышленном применении катализаторов и располагают достаточным временем для проведения эффективных занятий. Как будет показано ниже, одним из недостатков университетских курсов в Италии является недостаточное внимание, уделяемое промышленным аспектам катализа (промышленные формы катализаторов, дизайн каталитических реакторов и процессов, способы организации промышленного производства с участием каталитических процессов).
В докладе С.В. Джонса сообщается, что в США катализ, и особенно гетерогенный катализ, как предмет преподается в большей степени химикам-технологам, в то время как для химиков элементы кинетики и катализа введены в состав курсов по физической химии, органической химии и других базовых курсов. Отмечено также, что многие студенты не стремятся получить диплом магистра, а вместо этого поступают в аспирантуру непосредственно после бакалавриата, поскольку образовательная система США, в отличие от России, предусматривает такую возможность. Если же аспирант по какой-то причине не хочет продолжать работу над диссертацией, он защищает полученные в течение неполного срока обучения в аспирантуре результаты в качестве магистерской диссертации. Кроме того, обучение на степень магистра интересно тем студентам, которые заранее знают место будущей работы и могут выбрать соответствующие курсы. Было отмечено, что поступающие в аспирантуру студенты лишены возможности выбирать научного руководителя, они выбирают только научное направление для работы, а далее специальный совет при университете назначает им руководителей. Напротив, поступление “пост-доков” происходит непосредственно в научные группы, что следует учитывать при направлении российских ученых для обучения в аспирантуре и докторантуре США.
Итальянский преподаватель Ф. Кавани из Университета Болоньи при подготовке доклада провел интереснейший опрос выпускников университетов Италии (аспирантов и пост-доков) о качестве подготовки специалистов в области катализа. Анализ результатов опроса выявил недостаточность экспериментальной подготовки (менее 50% опрошенных проводили соответствующие эксперименты в ходе обучения). Примерно 40% опрошенных не видели катализатора в промышленном виде и не посещали во время обучения предприятия, использующие катализ. Примерно 12% опрошенных считают катализ эмпирической наукой, а 50% уверены, что предсказание каталитических свойств возможно в определенной степени. Важным и полезным для общества считают катализ более 90% опрошенных. Примерно 50% посещали занятия по катализу в ходе обучения в аспирантуре, еще 30% при обучении на магистра, остальные в бакалавриате. Таким образом, в целом обучение в области катализа в Италии можно считать успешным, поставленные цели достигаются.
Представитель Австралии Д. Белтрамини отметил практически полную невостребованность специалистов-каталитиков в своей стране, как и других специалистов для промышленности, в связи с преимущественно сырьевой и сервисной ориентацией австралийской экономики. Поэтому университеты Австралии готовят в год незначительное количество химиков-каталитиков (до двух десятков), причем они находят работу преимущественно за рубежом и там же продолжают при необходимости свое образование. Отметим, что только в Новосибирском университете выпускается не менее 17 магистров – специалистов в области катализа в год, а ведь такие специалисты проходят также подготовку во многих других университетах России – в Москве, Казани, Томске, Омске, Самаре, Твери и др.
В большинстве докладов подчеркивалось, что катализ входит в тематику “зеленой химии” и включен в соответствующие курсы, и наоборот. Так, Ф. Кавани (Италия) среди важнейших тем при обучении катализу называет использование катализа для превращения биомассы в химические продукты, так как именно это направление дает перспективы для получения энергии и химических материалов в будущем, по мере исчерпания запасов ископаемого топлива; М. Скоглунд (Швеция), перечисляя названия курсов по катализу в своем университете, отмечает курс “Зеленой химии”; курс лекций “Катализ и устойчивое развитие” упомянут В.И. Бухтияровым (Россия) как один из специальных курсов по катализу, читаемых в Новосибирском государственном университете. Аналогичный по содержанию курс назван Э. Хансеном среди курсов по выбору для аспирантов.
Вопросам экологического катализа уделяется значительное внимание при преподавании катализа в частном университете Чалмерс (Швеция). Помимо курса по “зеленой” химии, в нем преподают курс каталитического регулирования выбросов в окружающую среду, курс нанотехнологий для устойчивого производства энергии, эти вопросы также составляют существенную часть курса “Технологии двигателей внутреннего сгорания”. В докладе профессор этого университета М. Скоглунд представил четыре учебника, изданные в университете для его студентов (около 11000 человек), в их числе учебник по зеленой химии, которого так не хватает в России.
Изданием учебников по катализу также гордится университет Або/Турку (Финляндия), как отмечено в докладе профессора этого университета Д.Ю. Мурзина. В то же время в докладе Э. Хансена подчеркивалось отсутствие подходящего для обучения бакалавров и магистров учебника, поскольку существующие слишком глубоко специализированы на какой-либо определенной теме. По мнению докладчика, идеальный учебник должен включать разделы о приготовлении/характеризации/испытаниях катализатора и главы, характеризующие основные каталитические процессы; при этом учебник должен соединять атомистическое описание с химико-технологическим подходом и учить разномасштабному мышлению.
Необходимо подчеркнуть, что в России за последнее время издан только один краткий учебник по катализу профессора МГУ имени М.В. Ломоносова Б.В. Романовского “Основы катализа”. Он как раз ориентирован на базовое знакомство с предметом. Преимущественно обучение ведется в России по переводным учебникам (И. Чокендорф, Х. Наймантсведрайт “Современный катализ и химическая кинетика”, издан в 2007 г., переведен в 2010 г.) или по довольно старым книгам (например, классический труд О.В. Крылова по гетерогенному катализу). В то же время, при разработке учебных курсов в России требуется указывать в качестве учебных пособий свежую литературу, желательно выпущенную за последние пять лет; поэтому необходимо всячески поддерживать начинания, связанные с написанием российских учебников по катализу и зеленой химии.
Интересный подход к обучению в области катализа (курс “Реакционная кинетика и катализ”) применяется в университете Эйндховена (Нидерланды), прославленном именами Р. Ван Сантена и Х. Наймантсведрайта. Этот подход, как показано в докладе Э. Хенсена, заключается в “переворачивании” типов деятельности, характерных для аудиторной и самостоятельной подготовки. Студенты изучают он-лайн лекционные курсы, а аудиторные занятия служат для повторения основных пунктов, углубления знаний, выполнения упражнений. При подготовке магистров обязательный курс катализа проходят обучающиеся на химиков-технологов (курс “Катализ: наука и технологии”). В ходе выполнения курса студентам предлагается написать эссе на тему, связанную с катализом, например, по солнечным батареям. Для химиков обязательным является курс “Современная неорганическая химия”, включающий основы катализа. Также в качестве курса по выбору предлагается тематика “Современные концепции катализа”. При обучении аспирантов в Голландском институте каталитических исследований предлагается обязательный интегрированный курс по катализу с выдачей сертификата после экзамена. Для аспирантов каждые два года читаются также специальные курсы “Способы характеризации катализаторов”, “Современные каталитические технологии”, “Гомогенный катализ” и “Химия поверхности в катализе”, а также “Представление науки”. В середине 2016 года планируется выпуск в издательстве Elsevier учебника для аспирантов “Катализ: интегрированный подход”. Проводится школа по этой тематике, в которой принимает участие более 60 аспирантов, причем в течение одной недели обучение проходит на уединенном острове на севере Голландии, а далее аспиранты должны пройти несколько обязательных и курсов по выбору.
В нескольких докладах подчеркивалась важность освоения принципов промышленного катализа в сочетании с принципами “зеленой” химии. Так, в современном катализе предпочтительны и играют существенную роль полифункциональные катализаторы, а также концертные механизмы катализа, позволяющие задавать правильную последовательность стадий каталитического процесса в присутствии полифункционального катализатора. В связи с этим можно отметить, что исследованию таких катализаторов и механизмов их действия посвящено значительное количество представленных докладов российских участников Симпозиума.
Особенность России состоит в изобилии ископаемых ресурсов, значительном развитии нефтехимии и необходимости углублять переработку нефти, газа, угля с целью получения более выгодных в экономическом отношении продуктов. В докладах казанской научной школы уделялось внимание вопросам усовершенствования обучения специалистов-каталитиков для нефтехимической промышленности (доклад М.В. Журавлевой, О.В. Зиннуровой, Н.Ю. Башкирцевой (Казанский национальный исследовательский технологический университет, Россия) “Усовершенствованное обучение специалистов в области катализа в нефтехимической промышленности”, а также упомянутый выше доклад Х. Харлампиди и Г. Елимановой).
На других заседаниях Симпозиума были представлены доклады по тем областям науки о катализе, которые необходимо освещать в образовательных курсах. Ряд докладов показывал возможности использования методических подходов и экспериментальных установок, применяемых при проведении исследований, для практикумов по катализу (например, доклады А.М. Сульман, М.Г. Сульман, Е.М. Сульман, В.Ю. Долуда, Т.В. Лакиной (Россия) “Новый тип катализаторов для гидролитического гидрирования целлюлозы” и Н.В. Громова, О.П. Таран, М.Н. Тимофеевой, Е.Г. Жижиной, Ю.А. Родиковой, В.Н. Пармона (Россия) “Одностадийный метод для синтеза in situ аэрогелей из многостенных углеродных нанотрубок”).
Заметный вклад в программу внесли работы, посвященные разработке каталитических реакций и катализаторов, изучению механизмов каталитического действия и состава активных центров для превращения биомассы и ее компонентов в востребованные химические продукты или для получения энергии. Например, гетерогенный каталитический процесс превращения целлюлозы в 5-гидроксиметилфурфураль обсуждался в докладе Р.И. Хуснутдинова и др. Полифункциональный катализатор для превращения полисахаридов в полиолы представлен в докладе Ю.Н. Бигловой и др.
Отмечалась необходимость включения задач и практических занятий, связанных с экологическим катализом, в университетские курсы подготовки специалистов. Вопросы, связанные с очисткой выхлопных газов, поднимались, например, в докладах C. Hahn и др. “Insights into the elementary kinetics of the low-temperature NOX reduction by H2 on Pt/WO3/ZrO2 under lean conditions” и “Jeong Young Eun и др. Sulfated Sb-V-CeO2/TiO2 Catalyst Redox Features In The NH3-Scr Mechanism At Low Temperatures Investigated By Dynamic Ft-Ir”.
Заметная часть представленных докладов была посвящена вопросам фотокатализа и получения чистого водорода.
В заключение можно отметить, что сравнение методик и способов преподавания научных и практических аспектов каталитической науки в высшей школе оказалось исключительно плодотворным и выявило достоинства и слабые места преподавания в России и за рубежом. В дискуссии подчеркивалось желание и потребность в координации преподавания и обмене опытом в этой области. Прозвучало предложение о создании специального интернет-ресурса, например, в рамках EFCATS, для размещения и взаимного знакомства с образовательными материалами, методиками, учебниками, методическими пособиями в тех случаях, когда такие материалы не попадают под действие авторского права.
Участники Симпозиума сформулировали следующие заключительные пожелания:
1. Рекомендовать включать симпозиумы или круглые столы, посвященные образованию в области катализа, в программу следующих европейских конгрессов по катализу.
2. Обратиться в редакцию журнала ACS Catalysis с предложением выпустить специальный номер, посвященный этой тематике.
3. Создать специальный сайт или раздел сайта Европейской ассоциации каталитических обществ, на котором можно размещать в свободном доступе новые образовательные материалы, методики, учебники, методические пособия с целью обмена между всеми заинтересованными образовательными учреждениями.
Материал подготовили
Е.С. Локтева, В.В. Лунин (МГУ, Москва)
Д.Ю. Мурзин
(Университет Або/Турку, Финляндия)














Stereochemistry: Team grows homochiral crystals despite expectations
Scientists have long thought that chiral molecules could only form pure, orderly chiral crystals if those molecules all have the same chirality. Virgil Percec of the University of Pennsylvania and coworkers have now defied that long-established notion about the need for homochiral building blocks by growing high-purity supramolecular crystals from racemic and diastereomeric mixtures of perylene bisimides (PBIs) (Nat. Chem. 2015, DOI: 10.1038/nchem.2397).
 Racemic
and diastereomeric mixtures of PBIs (left, with chiral center in each
R group) self-assemble into columnar supramolecular helices (right),
which interlock with others by a cogwheel-like mechanism to form
homochiral crystals.
Racemic
and diastereomeric mixtures of PBIs (left, with chiral center in each
R group) self-assemble into columnar supramolecular helices (right),
which interlock with others by a cogwheel-like mechanism to form
homochiral crystals.
In a few past studies, research groups had shown that enantiomerically mixed polymers or supramolecular assemblies could separate into or form enantioenriched domains or crystals, but the products were not chirally pure or highly ordered. The new crystals, on the other hand, form when mixtures of PBIs stack on top of one another, self-assembling into chiral supramolecular helices. These columnar structures then combine to form pure homochiral crystals.
“This is a very significant discovery challenging the accepted view that homochirality is an essential prerequisite for self-assembly to form high-quality homochiral supramolecular crystals,” says Boris Rybtchinski, an expert on organic self-assembly at the Weizmann Institute of Science. The findings suggest that advanced homochiral materials for organic light-emitting diodes, spintronics, and other applications “could be achieved in a cost-effective way, starting from readily available nonhomochiral mixtures instead of very expensive, pure enantiomers.”
Percec and his team propose that their PBIs form homochiral crystals because the columnar helices that initially form by self-assembly have alkyl chains around their outsides that interlock in a cogwheel-like manner with chains on neighboring helices, which go on to interlock with other helices. Each PBI has six stereocenters, which end up facing inward toward the central axes of the columnar helices. Those stereocenters therefore don’t interfere with the interlocking process, making their configurations inconsequential to crystal formation.
Although the researchers observed the phenomenon on just one series of PBIs, the proposed mechanism suggests that it might be possible to extend the concept to other nonhomochiral building blocks with similar molecular features, says Ricardo Riguera of the University of Santiago de Compostela, whose interests include controlled chirality.
Supramolecular systems specialist E.W. (Bert) Meijer of Eindhoven University of Technology calls the study “remarkable.” It shows “that mixtures of molecules with even six stereocenters and all possible chiralities always give one type of supramolecular product,” he says. “This is unexpected for many in the field. The results give new insights into the amplification of chirality in large supramolecular structures and could be important for understanding the origins of homochirality in nature.”
Climate Change: Pledges for new treaty aren’t enough to restrain temperature rise to 2 °C
Concentrations of greenhouse gases in the atmosphere continue to break records, with global average carbon dioxide concentrations reaching 397.7 parts per million in 2014, the World Meteorological Organization (WMO) says.
 The annual
average carbon dioxide concentration worldwide is approaching 400
parts per million. SOURCE: World
Meteorological Organization
The annual
average carbon dioxide concentration worldwide is approaching 400
parts per million. SOURCE: World
Meteorological Organization
“We will soon be living with globally averaged CO2 levels above 400 ppm as a permanent reality,” says WMO Secretary-General Michel Jarraud.
Two other key greenhouse gases also set records in 2014. Methane levels were 1,833 parts per billion and concentrations of nitrous oxide reached 327 ppb, says WMO, the United Nations agency considered the world’s scientific authority on Earth’s atmosphere.
Radiative forcing—a warming effect—from greenhouse gases increased 36% between 1990 and 2014, WMO says. The organization points out that warming caused by climbing CO2 concentrations has led to an increase in the level of water vapor, which is also a greenhouse gas. Warmer air holds more moisture, WMO explains.
“We have to act now to slash greenhouse gas emissions if we are to have a chance to keep the increase in temperatures to manageable levels,” Jarraud warns.
Global diplomatic talks to cut those emissions are under way and are expected to produce a new climate change treaty in Paris next month. Thus far, 160 countries have individually pledged to carry out specific actions to control emissions.
But a scientific assessment of those pledges, conducted by the United Nations Environment Programme (UNEP), says they fall short of what’s needed to hold the global temperature rise to 2 °C by 2100 when compared with preindustrial levels.
Annual global greenhouse gas emissions need to be equivalent to 42 billion metric tons of CO2 in 2030 for at least a 66% chance of meeting that policy goal, UNEP says. Full implementation of all pledges made thus far would lead to emissions equivalent to 52 to 57 billion metric tons of CO2 in 2030, the agency estimates. This level would put the world on track for a temperature rise of about 3 °C by the end of the century, UNEP adds.
Materials Science: Liquid made with cage compounds has permanent pores and could one day help with gas separations
To generate a holey liquid (bottle), researchers dissolved a porous core structure (space-filling) with crown-ether decorations (ball and stick) in a crown-ether solvent.
Stuart James of Queen’s University Belfast and coworkers have devised a liquid with permanent porosity (Nature 2015, DOI: 10.1038/nature16072). Such holey fluids could be useful in gas separation, process chemistry, and other applications, if they can be made economically. Their persistent porosity comes from hollow organic cage molecules coated with solvent-soluble surface groups. The cage openings are too small to be clogged by the surface groups or by large solvent molecules in the surrounding solvent.
Liquids contain spaces between their molecules, but they are tiny. Bubbles can be blown into liquids, but they will quickly float to the surface and dissipate—air bubbles in glass being a rare exception.
On the other hand, solids such as zeolites and metal-organic frameworks have permanent pores. They can be used to separate molecules by size and can host added catalysts to drive chemical reactions. But unlike liquids, these materials can’t flow through channels or be smoothed onto surfaces.
James got the idea for holey liquids a decade ago, when a colleague, Queen’s University chemical engineer David W. Rooney, wondered whether mixtures of porous solids and liquids might be pumped through pipes and used in continuous processes more easily than is generally possible with porous solids alone.
Porous solids expert Russell Morris of the University of St. Andrews comments that the concept of liquids with permanent porosity has actually been around for decades, but “the practicalities of designing such a system take skill and perseverance. This work delivers real liquids that show that property, which is a great achievement.”
The researchers initially achieved liquid porosity by dissolving a hollow organic core structure coated with crown-ether surface groups in a crown-ether solvent. To optimize gas absorption capacity, the team packed the fluid with as many cage molecules as possible—one for every 12 solvent molecules. When exposed to methane, the liquid absorbs eight times as much gas as the solvent alone.
But the crown ethers were hard to synthesize and the liquid thick and slow-flowing. So collaborators Andrew I. Cooper and Rebecca L. Greenaway at the University of Liverpool developed another porous liquid by coating a hollow organic cage with a mixture of diamines and dissolving it in the solvent hexachloropropene. The resulting porous liquid flows ten times as readily as the crown-ether-based material. The researchers synthesize the coated cages in a single step, and the solvent is available commercially.
In earlier work, Shannon M. Mahurin and Sheng Dai at Oak Ridge National Laboratory and coworkers created hollow colloidal silica nanoparticles with a fluidlike polymer surface coating (Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201409420), but the new porous liquids are easier to molecularly modify. Dai comments that “the liquids will open up new frontiers in how we think about porosity.”
The surface area and gas uptake of the new materials are too modest for them to compete with porous solids immediately, says cage compound specialist Michael Mastalerz of Germnay’s Heidelberg University in a Nature commentary. “They should instead be seen as a prototype of a new class of material [that] will undoubtedly find technological applications” if these properties can be improved.
Materials: Cages made from nanoparticles could challenge dominance of ELISA enzyme
Placing copper hydroxide nanoparticles in a copper-ammonia solution causes the particles to transform into nanoribbons, which become the bars of a cage with an empty center.
Researchers have been exploring ways to build tiny containers out of nanoparticles; such superstructures can help deliver drugs to specific tissues, hold molecules that act as biological sensors or catalyze reactions, and make it easier for microscopes or MRI machines to image tiny targets. Now, one group has come up with an easy way to build nanoparticle cages, and to their surprise, the boxes are much more efficient catalysts than an enzyme commonly used in ELISA, the popular immunoassay for detecting many types of biomolecules (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b09337).
Weihong Tan, a chemist at the University of Florida and Hunan University, in China, and his group were working to create large-surface-area nanostructures that could hold and deliver high doses of drugs. Existing processes for making such superstructures are time consuming and complex, and the researchers were hoping their approach would be simpler.
The team started by making copper hydroxide nanoparticles, which they dispersed in water with polyvinylpyrrolidone. Next, they added a copper-ammonia complex to the solution, triggering a series of reactions that led to migration of the copper hydroxide into one-dimensional “nanoribbons” that formed evenly spaced bars surrounding the nanoparticles. Eventually the 200- to 250-nm particles completely disappeared, leaving behind a self-assembled three-dimensional cage of the same size with a hollow center. “The formation is very regular, and it gives you a uniform structure,” Tan says. He does not yet have an explanation for the mechanism leading to that structure.
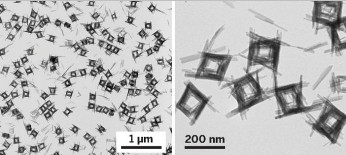
Transmission electron micrographs show cages formed from spontaneous reshaping of copper hydroxide nanoparticles.
The group tested the material’s catalytic activity and found that it had high activity for the colorimetric reaction typically catalyzed by the horseradish peroxidase enzyme. This reaction is central to many biological assays, especially the widely used ELISA, or enzyme-linked immunosorbent assay, used to detect the presence of antigens in samples. In the presence of the group’s cages, a solution containing the reagents tetramethylbenzidine and hydrogen peroxide changed from clear to deep blue within five minutes at room temperature. The same reaction with horseradish peroxidase took longer, even though the concentration of the enzyme was 2,000 times that of the copper hydroxide cages. Tan says he needs to do more work to figure out how the system works. “I don’t really know why we have all this catalytic activity,” he says.
Jianfang Wang, a professor of physics who studies nanoparticles at the Chinese University of Hong Kong, is impressed with the work. “I am very surprised by the fact that such complex nanoarchitectures can be synthesized by such a simple wet-chemistry method in aqueous solutions,” he says. “This is probably the most complex structure that I have ever seen among those made by wet-chemistry methods.”
He would like to know more about the mechanism that causes the cages to form, as well what causes the high catalytic activity. He’s also interested in learning how selective the material is. “Selectivity is probably more important than activity for enzymes,” Wang says.
Tan says his next step is to see whether he can make a complete assay that might be able to replace ELISA.
Chemical & Engineering News
ChemCatChem
The Direct Non-Perturbing Leaching Test in the Phosphine-Free Suzuki–Miyaura Reaction Catalyzed by Palladium Nanoparticles
Alexander N. Kashin, Olga G. Ganina, Andrey V. Cheprakov, Irina P. Beletskaya
http://onlinelibrary.wiley.com/doi/10.1002/cctc.201500329/full
Advanced Synthesis & Catalysis
The Nitro to Carbonyl Conversion (Nef Reaction): New Perspectives for a Classical Transformation
Roberto Ballini, Marino Petrini
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/adsc.201500008/
?elq_mid=5863&elq_cid=1938826
ChemElectroChem
Ternary Transition Metal Oxide Nanoparticles with Spinel Structure for the Oxygen Reduction Reaction
Rou Jun Toh, Alex Yong Sheng Eng, Zdenek Sofer, et al.
http://onlinelibrary.wiley.com/doi/10.1002/celc.201500070/full
Angewandte Chemie International Edition
Electrodeposited Cobalt-Phosphorous-Derived Films as Competent Bifunctional Catalysts for Overall Water Splitting
Nan Jiang, Bo You, Meili Sheng, Yujie Sun
http://onlinelibrary.wiley.com/doi/10.1002/anie.201501616/full
Chemistry – A European Journal
Enhancing Effects of Salt Formation on Catalytic Activity and Enantioselectivity for Asymmetric Hydrogenation of Isoquinolinium Salts by Dinuclear Halide-Bridged Iridium Complexes
Bearing Chiral Diphosphine Ligands
Yusuke Kita, Kenta Yamaji, Kosuke Higashida, et al.
http://onlinelibrary.wiley.com/doi/10.1002/chem.201405408/full
Macromolecular Chemistry and Physics
Highly Efficient and Reusable Microporous Schiff Base Network Polymer as a
Heterogeneous Catalyst for CuAAC Click Reaction
Omer Suat Taskin, Sajjad Dadashi-Silab, Baris Kiskan, Jens Weber, Yusuf
Yagci
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/macp.201500141
Energy Technology
Heterogeneously-Catalyzed Hydrogenation of Carbon Dioxide to Methane using RuNi Bimetallic Catalysts
Felix Lange, Udo Armbruster, Andreas Martin
http://onlinelibrary.wiley.com/doi/10.1002/ente.201402113/full
ChemPhysChem
Vacuum Surface Science Meets Heterogeneous Catalysis: Dehydrogenation of a Liquid Organic Hydrogen Carrier in the Liquid State
Takashi Matsuda, Nicola Taccardi, Johannes Schwegler, et al.
http://onlinelibrary.wiley.com/doi/10.1002/cphc.201500236/full
ChemPlusChem
Utilizing Benign Oxidants for Selective Aerobic Oxidations Using Heterogenized Platinum Nanoparticle Catalysts
Christopher S. Hinde, Arran M. Gill, Peter P. Wells, T. S. Andy Hor, Robert Raja
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/cplu.201500195
Chemistry – An Asian Journal
Iodine-Catalyzed Oxidative Coupling Reactions Utilizing C—H and X—H as Nucleophiles
Dong Liu, Aiwen Lei
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/asia.201403248
Applied Organometallic Chemistry
Pd(II) complexes of Schiff bases and their application as catalysts in Mizoroki–Heck and Suzuki–Miyaura cross-coupling
reactions
Mustafa Keleş, Hülya Keleş, Duygu Melis Emir<
http://onlinelibrary.wiley.com/enhanced/doi/10.1002
Journal of Physical Organic Chemistry
The relationships of catalytic activity of metal Schiff base catalysts
and the Hammett constants of their anion–cationic substituents on ligand
Hang Chen, Lu Jia, Jia Yao, et al.
http://onlinelibrary.wiley.com/enhanced/doi/10.1002
| 1 | ACS Applied Materials and Interfaces | 6.723 |
| 2 | ACS Catalysis | 9.312 |
| 3 | Adsorption | 1.771 |
| 4 | Adsorption Science and Technology | 0.669 |
| 5 | Advanced Powder Technology | 2.638 |
| 6 | Advanced Synthesis and Catalysis | 5.663 |
| 7 | Advances in Catalysis | 10 |
| 8 | Angewandte Chemie - International Edition | 11.261 |
| 9 | Applied Catalysis A: General | 3.942 |
| 10 | Applied Catalysis B: Environmental | 7.435 |
| 11 | Applied Organometallic Chemistry | 2.248 |
| 12 | Applied Physics A: Materials Science and Processing | 1.704 |
| 13 | Applied Physics Letters | 3.302 |
| 14 | Bulletin of Materials Science | 1.017 |
| 15 | Carbon | 6.196 |
| 16 | Catalysis Communications | 3.699 |
| 17 | Catalysis in Industry | 0.596 |
| 18 | Catalysis Letters | 2.307 |
| 19 | Catalysis Reviews: Science and Engineering | 8.471 |
| 20 | Catalysis Science and Technology | 5.426 |
| 21 | Catalysis Today | 3.893 |
| 22 | ChemCatChem | 4.556 |
| 23 | Chemical Communications | 6.834 |
| 24 | Chemical Engineering and Technology | 2.442 |
| 25 | Chemical Engineering Journal | 4.321 |
| 26 | Chemical Engineering Research and Design | 2.348 |
| 27 | Chemical Engineering Science | 2.337 |
| 28 | Chemical Physics | 1.652 |
| 29 | Chemical Physics Letters | 1.897 |
| 30 | Chemical Reviews | 46.568 |
| 31 | Chemical Society Reviews | 33.383 |
| 32 | Chemistry - A European Journal | 5.731 |
| 33 | Chemistry - An Asian Journal | 4.587 |
| 34 | Chemistry and Technology of Fuels and Oils | 0.133 |
| 35 | Chemistry of Materials | 8.354 |
| 36 | ChemPhysChem | 3.419 |
| 37 | ChemSusChem | 7.657 |
| 38 | Colloid Journal of the Russian Academy of Sciences: Kolloidnyi Zhurnal | 0.789 |
| 39 | Cuihua Xuebao / Chinese Journal of Catalysis | 1.964 |
| 40 | Doklady Chemistry | 0.413 |
| 41 | Doklady Physical Chemistry | 0.586 |
| 42 | Electrocatalysis | 2.367 |
| 43 | Electrochemistry Communications | 4.847 |
| 44 | Fuel Cells | 2.08 |
| 45 | International Journal of Chemical Kinetics | 1.517 |
| 46 | International Journal of Nanotechnology | 0.618 |
| 47 | International Journal of Quantum Chemistry | 1.432 |
| 48 | Journal of Alloys and Compounds | 2.999 |
| 49 | Journal of Catalysis | 6.921 |
| 50 | Journal of Chemical Physics | 2.952 |
| 51 | Journal of Chemistry | 0.361 |
| 52 | Journal of Environmental Engineering | 1.267 |
| 53 | Journal of Fuel Cell Science and Technology | 0.858 |
| 54 | Journal of Hazardous Materials | 4.529 |
| 55 | Journal of Materials Chemistry A, Materials for Energy and Sustainability | 7.443 |
| 56 | Journal of Molecular Catalysis A: Chemical | 3.615 |
| 57 | Journal of Molecular Catalysis B: Enzymatic | 2.128 |
| 58 | Journal of Organometallic Chemistry | 2.173 |
| 59 | Journal of Structural Chemistry | 0.508 |
| 60 | Journal of the American Chemical Society | 12.113 |
| 61 | Journal of the European Ceramic Society | 2.947 |
| 62 | Kinetics and Catalysis | 0.758 |
| 63 | Langmuir | 4.457 |
| 64 | Materials Letters | 2.489 |
| 65 | Mendeleev Communications | 1.34 |
| 66 | Microporous and Mesoporous Materials | 3.453 |
| 67 | PCCP: Physical Chemistry Chemical Physics | 4.493 |
| 68 | Reaction Kinetics, Mechanisms and Catalysis | 1.17 |
| 69 | Russian Chemical Reviews | 2.318 |
| 70 | Russian Journal of Applied Chemistry | 0.276 |
| 71 | Tetrahedron | 2.641 |
| 72 | The Journal of Physical Chemistry A | 2.693 |
| 73 | Topics in Catalysis | 2.365 |
October 2-6, 2016 (Svetlogorsk, Kaliningrad region, Russia)
The X International Conference “Mechanisms of Catalytic Reactions” (MCR-X) will be held in Svetlogorsk (Kaliningrad region, Russia) on 2-6 October, 2016. The Conference is organized under the auspices of the European Federation of Catalysis Societies and the National Catalytic Society of Russia.
The Conference is the 10th event in the series of International MCR Conferences started in 1974 in Moscow. Traditionally, the International Conferences on the Mechanisms of Catalytic Reactions focus on advances in understanding the mechanisms of chemical reactions occurring in the presence of catalysts, ranging from homogeneous molecular catalysts (inorganic, organic, metal complex based) to heterogeneous catalysts.
| Valentin P. Ananikov | Zelinsky Institute of Organic Chemistry RAS, Moscow, Russia |
| Miguel A. Bañares | ICP-CSIC, Madrid, Spain |
| Alexis T. Bell | University of California, Berkeley, USA |
| Konstantin P. Bryliakov | Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia |
| Valerii I. Bukhtiyarov | Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia |
| Irina I. Ivanova | Topchiev Institute of Petrochemical Synthesis RAS, Moscow, Russia |
| Vasily V. Kaichev | Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia |
| Ekaterina A. Kozlova | Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia |
| Valery V. Lunin | Lomonosov Moscow State University, Moscow, Russia |
| Ilya I. Moiseev | Gubkin Russian State University of Oil and Gas, Moscow, Russia |
| Dmitry Yu. Murzin | Abo Akademi University, Turku, Finland |
| Konstantin M. Neyman | ICREA & University of Barcelona, Barcelona, Spain |
| Emrah Ozensoy | Bilkent University, Ankara, Turkey |
| Valentin N. Parmon | Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia |
| Gunther Rupprechter | Vienna University of Technology, Vienna, Austria |
| Philippe Sautet | University of Lyon, Lyon, France |
| Robert Schlögl | Fritz Haber Institute of the Max Planck Society,Max Planck Institute for Chemical Energy Conversion, Germany |
| Mikhail Yu. Sinev | Semenov Institute of Chemical Physics RAS, Moscow, Russia |
| Malgorzata Witko | Institute of Catalysis and Surface Chemistry, Krakow, Poland |
| Chairman: V.I. Bukhtiyarov | Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia |
| Vice hairman: A.V. Yurov | Immanuel Kant Baltic Federal University, Kaliningrad, Russia |
| Members: | |
| K.P. Bryliakov | Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia |
| А.А. Gribankova | Immanuel Kant Baltic Federal University, Kaliningrad, Russia |
| V.V. Kaichev | Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia |
| A.A. Karasik | Arbuzov Institute of Organic and Physical Chemistry RAS, Kazan, Russia |
| E.A. Kozlova | Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia |
| V.N. Leitsin | Immanuel Kant Baltic Federal University, Kaliningrad, Russia |
| E.S. Lokteva | Lomonosov Moscow State University, Moscow, Russia |
| A.A. Saraev | Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia |
| V.V. Savin | Immanuel Kant Baltic Federal University, Kaliningrad, Russia |
| A.Yu. Stakheev | Zelinsky Institute of Organic Chemistry RAS, Moscow, Russia |
| L.Ya. Startseva | Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia |
| O.V. Turova | Zelinsky Institute of Organic Chemistry RAS, Moscow, Russia |
| Secretariat: | |
| А.A. Eremenko | Immanuel Kant Baltic Federal University, Kaliningrad, Russia |
| M.A. Klyusa | Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia |
| D.A. Omelyanovich | Immanuel Kant Baltic Federal University, Kaliningrad, Russia |
The scientific program of MCR X includes four plenary lectures (40 min) and seven keynote lectures (30 min). Oral presentations (20 min) are scheduled in 2 parallel sessions. The Conference Program also includes poster presentations. The main topics are:
The Conference will be accompanied by the School-Seminar “Powder materials in catalytic processes: applications, methods of extraction, structure and properties” and the School-Symposium “Quantum-mechanical modeling of catalytic processes”.
The official language of the Conference is English.
Leading scientists will give plenary and keynote lectures:
Plenary lecturers
Confirmed Keynote speakers
Abstract Submission
The final Scientific Program brochure and Book of Abstracts of lectures, oral and poster presentations on a USB key will be available at the registration desk.
Authors of all contributions are invited to submit one-page abstracts (A4) in English via the MCR-X official website http://conf.nsc.ru/MCR-X/en before February 15, 2016.
Key dates
| February 15, 2016 | Submission of preliminary registration forms and Abstracts |
| April 30, 2016 | Notification of acceptance |
| June 1, 2016 | Distribution of preliminary Scientific Program |
| June 15 – August 30, 2016 | Payment of fee |
| October 2, 2016 | Arrival of participants |
| October 2-5, 2016 | Working days |
| October 6, 2016 | Post-tour |
For information please contact:
| In Novosibirsk: | In Kaliningrad: |
|
Liudmila Startseva Boreskov Institute of Catalysis E-mail: star@catalysis.ru tel./fax: +7(383)330-62-97 |
Anastasiya Eremenko Immanuel Kant Baltic Federal University E-mail: AAEremenko@kantiana.ru tel.: +7 (4012) 595 595 (ext. 7865) |
15-20 мая 2016 г. (Омск, Россия)
Приглашаем Вас принять участие в V Всероссийской научной молодежной школе-конференции «Химия под знаком СИГМА: исследования, инновации, технологии», которая состоится 15-20 мая 2016 г. в городе Омске. В рамках школы-конференции традиционно будут представлены пленарные лекции ведущих ученых-химиков, устные и стендовые доклады молодых ученых, специалистов и преподавателей по современным направлениям фундаментальных и прикладных исследований в области химии и химической технологии.
Член-корр. РАН В.А. Лихолобов (председатель)
Академик В.Н. Пармон
Член-корр. РАН В.И. Бухтияров
Д.х.н. Б.Н. Кузнецов
Д.х.н. А.В. Мышлявцев
Д.х.н. А.С. Белый
Д.х.н. П.Г. Цырульников
Д.х.н. А.С. Фисюк
Д.х.н. В.И. Вершинин
К.х.н. А.В. Лавренов
К.х.н. А.А. Ведягин
К.х.н. Д.А. Шляпин
К.х.н.
Л.Н. Степанова (председатель)
К.ф.-
м.н. Д.Ф. Хабибулин
К.х.н.
В.А. Борисов
К.х.н.
А.В. Седанова
К.х.н.
Н.С. Смирнова
Н.В.
Виниченко
М.С.
Дроздецкая
Е.С.
Запевалова
М.В.
Журавлева
А.А.
Непомнящий
П.Е.
Павлюченко
В.А.
Шкуренок
Ю.Е. Клепикова, В.Л. Юрпалов
Программа школы-конференции включает лекции ведущих ученых-химиков, устные доклады молодых ученых, стендовые доклады, тренинг-семинар «Интеграция».
1. Углеродные и неорганические материалы
2. Аналитическая химия и физико-химические методы исследования
3. Кинетика и катализ
4. Химическая технология
| 10 ноября – 25 декабря 2015 г. | Регистрация участников |
| до 1 февраля 2016 г. | Прием тезисов докладов |
| до 1 марта 2016 г. | Уведомление о принятии докладов |
| до 2 апреля 2016 г. | Рассылка информационного сообщения № 2 и научной программы школы-конференции |
| 15 мая 2016 г. | Начало работы школы-конференции |
644040, г. Омск, ул. Нефтезаводская, 54 (ИППУ СО РАН)
"Официальный e-mail конференции: chemsigma2016@gmail.com
(в теме письма указывать «Конференция
Сигма»)
Юрпалов Вячеслав Леонидович,тел. 8-983-563-86-88
| |
|
|
January 7-9, 2016 Nanoparticle Assembly: From Fundamentals to Applications. Faraday Discussion Mumbai, India |
http://www.rsc.org/ConferencesAndEvents/ RSCConferences/FD/Assembly-FD2016/index.asp |
|
25-27 января 2016 г.
Всероссийская научно-практическая конференция “Перспективы развития углехимической науки, углехимических технологий и углехимических производств в Российской Федерации” Кемерово, Россия |
(3842) 77 88 11 |
|
March
20-23, 2016 International Conference on Nanotechnology Based Innovative Applications for the Environment Rome, Italy |
http://www.aidic.it/nine |
|
April
3-6, 2016 Green and Sustainable Chemistry Conference Berlin, Germany |
http://www.greensuschemconf.com/ |
|
April
4-6, 2016 Designing New Heterogeneous Catalysis. Faraday Discussion London, UK |
http://www.rsc.org/ConferencesAndEvents/ RSCConferences/FD/Catalysis-FD2016/index.asp |
|
April
10-13, 2016 5th International Conference on Industrial Biotechnology (IBIC 2016) Bologna, Italy |
http://www.aidic.it/ibic2016 |
|
17-23 апреля 2016 г. II Научно-технологический симпозиум “Нефтепереработка: катализаторы и гидропроцессы” Белград, Сербия |
http://conf.nsc.ru/STS_2 |
|
19-20 апреля 2016 г. VI Всероссийская конференция с международным участием “Каучук и Резина - 2016: традиции и новации” Москва, Россия |
+7 (499) 256 21 66; +7 (916) 035 64 54 tkonikova@mail.ru http://www.rubberconference.ru/conf2016_1/ about/news/ZHdem_Vas_na_konferentsii_206_goda |
|
April
19-22, 2016 Carbon Dioxide Catalysis Conference Carvoeiro, Portugal |
http://www.zingconferences.com/conferences/ carbon-dioxide-catalysis |
|
May
2-6, 2016 2016 E-MRS Spring Meeting & Exhibit Lille, France |
http://www.european-mrs.com/meetings/2016-spring-meeting |
|
May
8-11, 2016 7th International Conference on Hydrogen Production (ICH2P 2016) Hangzhou, China |
http://ich2p-16.csp.escience.cn/dct |
|
11-13 мая 2016 г. VI Всероссийская конференция молодых ученых “Материаловедение, технологии и экология в третьем тысячелетии” Томск, Россия |
http://mte.iao.ru |
|
15-20 мая 2016 г. V Всероссийская научная молодежная школа-конференция “Химия под знаком СИГМА: исследования, инновации, технологии” Омск, Россия |
http://sigma.ihcp.ru |
|
May
22-26, 2016 7th International Symposium on Molecular Aspects of Catalysis by Sulfides (MACS-VII) Doorn, the Netherlands |
http://www.macs2016.com |
|
May
23-27, 2016 French Conference on Catalysis (FCCat 1) Frejus (French Riviera), France |
http://fccat.sciencesconf.org |
|
5-15 июня 2016 г. 10-я Международная конференция “Углерод: фундаментальные проблемы науки, материаловедение, технология” Троицк (Москва), Россия |
oalexandrovsky@yandex.ru |
|
June
13-17, 2016 9th European meeting on Solar Chemistry and Photocatalysis: Environmental Applications (SPEA) Strasbourg, France |
http://spea9.unistra.fr |
|
23-27 июня 2016 г. VIII школа-семинар молодых ученых России “Проблемы устойчивого развития региона” Истомино, оз. Байкал, около Улан-Удэ |
http://www.binm.ru school-seminar.binm8@mail.ru |
|
27 июня – 1 июля
2016 г. Кластер конференций по органической химии (ОргХим-2016) Репино, Санкт-Петербург, Россия |
http://onlinereg.ru/orgchem2016 |
|
June 30
– July 3, 2016 BIT's 7th Annual Global Congress of Catalysis 2016 (GCC-2016) Seoul, South Korea |
http://www.bitcongress.com/gcc2016 |
|
July
3-8, 2016 16th International Congress on Catalysis (ICC 16) Beijing, China |
www.icc2016china.com |
|
July
4-6, 2016 Nanoparticles with Morphological and Functional Anisotropy. Faraday Discussion Glasgow, UK |
http://www.rsc.org/ConferencesAndEvents/ RSCConferences/FD/Anisotropy-FD2016/index.asp |
|
July
10-13, 2016 9th International Conference on Environmental Catalysis (19th ICEC 2016) Newcastle, Australia |
www.icec2016.org |
|
July
10-15, 2016 Biocatalysis. Gordon Research Conference Biddeford, USA |
http://www.grc.org/programs.aspx?id=12255 |
|
September
19-22, 2016 2016 E-MRS Fall Meeting & Exhibit Warsaw, Poland |
http://www.european-mrs.com/meetings/2016-fall |
|
September
19-23, 2016 XXII International Conference on Chemical Reactors (CHEMREACTOR-22) London, UK |
http://conf.nsc.ru/CR_22 |
|
19-23 сентября 2016 г. XV Международная конференцияпо термическому анализу и калориметрии в России (RTAC-2016) Санкт-Петербург, Россия |
http://crtac.com |
|
26-30 сентября 2016 г. XX Менделеевский съезд по общей и прикладной химии Екатеринбург, Россия |
http://mendeleev2016.uran.ru |
|
October
2-6, 2016 X International Conference “Mechanisms of Catalytic Reactions” (MCR-X) Svetlogorsk, Kaliningrad region, Russia |
http://conf.nsc.ru/MCR-X |
|
November
27 – December 2, 2016 2016 MRS Fall Meeting & Exhibit Boston, USA |
http://www.mrs.org/fall2016 |
| | |
|
August
27 – September 1, 2017 11th Triennial Congress of the World Association of Theoretical and Computational Chemists (WATOC2017) Munich, Germany |
http://www.watoc2017.com |


