
Synthesis: Chemists turn cobalt (III) complexes into chiral hydrogen-bond-donor catalysts
|
A modified Co(III) Werner complex catalyzes the addition of malonate esters to nitroalkanes with up to 98% enantioselectivity. |
Chemists have tweaked a century-old, chiral cobalt complex to catalyze reactions via hydrogen bond donors on the ligands, rather than at the central metal.
John A. Gladysz, chemistry professor at Texas A&M University, College Station, and his colleagues think the new class of versatile and low-cost enantioselective catalysts may greatly broaden the options for synthesizing enantiomerically pure pharmaceuticals and agrochemicals.
Hydrogen bond donation catalysis is becoming increasingly popular as a strategy for controlling enantioselectivity. Previous work has focused on organic catalysts containing NH and OH groups that catalyze reactions by stabilizing transition states in specific orientations through hydrogen bonding.
“We have expanded this concept to a new, unexplored corner of the ‘chiral pool’ that has never been used for enantioselective catalysis,” Gladysz tells C&EN.
The group fitted a cobalt(III) Werner complex with 1,2-diphenylethylenediamine ligands, which catalyze reactions via their NH groups. They used the catalyst to perform a carbon-carbon bond-forming reaction, the Michael addition of malonate esters to nitroalkenes, with up to 98% enantioselectivity (ACS Cent. Sci. 2015, DOI: 10.1021/acscentsci.5b00035).
“This is a fundamentally important concept in utilizing … these complexes for asymmetric catalysis—a scaffold that had no prior catalytic applications,” says Thomas J. Colacot, global R&D manager for homogeneous catalysis at Johnson Matthey Catalysis & Chiral Technologies in West Deptford, N.J.
Also, since the complexes have 12 NH bonds to participate in the reaction, as opposed to just two for most current hydrogen bond donors, the group should be able to create new catalysts, Gladysz tells C&EN.
“This novel mode of ligand-centered catalysis … should inspire new catalyst design, as ancillary ligands are traditionally used to modulate reactivity at the metal center,” Colacot says.
Yuri Belokon, professor at the Russian Academy of Sciences’ A. N. Nesmeyanov Institute of Organoelement Compounds in Moscow, agrees. He predicts that within several years many groups will be employing hydrogen-bond-donating chiral cobalt(III) complexes in their reactions.
Materials: Atomic-scale imperfections act like a bucket line, passing protons through the material
|
Handoff Hydroxyl groups bonded to carbon atoms that form a tiny graphene defect pass protons in water from one OH group to another through a graphene film. O is red, H is white, C is gray, yellow marks proton starting position on a water molecule, and blue spheres indicate proton transfer route. |
Like an atomic-scale bucket brigade, molecular species residing at defects in graphene work together to shuttle protons through the ultrathin carbon film, according to a new study.
The investigation surprisingly shows that single layers of graphene, on their own, can selectively transmit protons in water. The finding deepens understanding of transport properties of the atomically thin carbon material and may lead to improved proton-selective membranes, a critical component of fuel cells.
In the ongoing push to explore graphene’s potential applications, several researchers have studied proton conduction through graphene. The results indicate that protons cannot pass through the material, unless researchers modify it with dopants, puncture it to form fine holes, or apply a voltage.
The new study, which was conducted by a multi-institution team led by Franz M. Geiger of Northwestern University, shows that those procedures are not required to coax protons through graphene (Nat. Commun. 2015, DOI: 10.1038/ncomms7539). Rather, a small number of atomic-scale defects that form naturally during graphene synthesis cause the material to rapidly transmit aqueous protons through the carbon network.
The team deposited a carefully characterized graphene film on a silica support and then added an aqueous solution. As they cycled the solution between low and high pH values, the team used a highly sensitive laser spectroscopy method to monitor protonation and deprotonation of silanol groups on the silica surface. When the solution pH was low, protons in solution moved through the graphene film to the silanol groups, and when the pH was high, protons traveled in the opposite direction. Through a combination of microscopy and other analyses, the group ruled out proton diffusion through pinhole defects and ensured that the film was not damaged by exposure to laser light and other probes.
The analysis, coupled with computations, shows that graphene exhibits rare defects—holes—surrounded by six carbon atoms that are either terminated with three oxygen atoms or six OH groups. The terminating oxygen atoms prevent proton transfer. But the hydroxyl groups work like an old-time bucket brigade grabbing protons from water and passing them quickly from one OH group to another, thereby transporting protons through the graphene membrane.
“The upshot is—for proton-separating membranes all you need is slightly imperfect single-layer graphene,” Geiger says.
Relative to earlier investigations of graphene proton transport, “this paper reports important technical and scientific advances,” says Mischa Bonn of the Max Planck Institute for Polymer Research, in Germany. The study, he says, provides insights into the proton conduction mechanism and describes a relay for protons that’s inaccessible to atoms and molecules.
Chemistry professor James T. (Casey) Hynes of the University of Colorado, Boulder, remarks that the study adds to the list of known proton relay chains, such as ones through proteins or at ice surfaces in the stratosphere. “This addition to the list, in an utterly hydrophobic environment, is a quite striking and pleasant surprise.”
Green Chemistry: Italian firms pursue development of intermediate from cellulosic feedstocks
Levulinic acid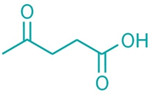 Two
Italian chemical firms have launched projects to produce levulinic acid from biomass. The companies say their technologies will lower
costs and turn a niche chemical into an attractive new building block for products used in crop protection, coatings, solvents, and fuels.
Two
Italian chemical firms have launched projects to produce levulinic acid from biomass. The companies say their technologies will lower
costs and turn a niche chemical into an attractive new building block for products used in crop protection, coatings, solvents, and fuels.
GFBiochemicals, based in Milan, says it will begin commercial production of levulinic acid from a starch feedstock this summer in Caserta, Italy. Capacity will start at 2,000 metric tons per year and scale up to 8,000 metric tons by 2017, the firm says. It aims to switch to cellulose-based feedstock in 2016. GFB’s executives, veterans of big companies such as Solvay and Air Products, say they have the expertise to sell the chemical for new applications where it can replace petroleum-derived inputs.
Meanwhile, Bio-on, a maker of biodegradable polyhydroxyalkanoate resins, says it will collaborate with the sugar company Eridania Italia to produce levulinic acid from sugar by-products. Eridania will invest roughly $2 million; the firms contend the molecule has immediate potential in biodegradable plastics.
Fans of levulinic acid would like to see it follow in the footsteps of succinic acid, another biobased intermediate that is being
commercialized by BioAmber, Myriant, and Reverdia. Both substances
were on
a
list of the 12 most promising chemicals from biomass put out a decade ago by the U.S. Department of Energy. But whereas
succinic acid is made from sugars and requires fermentation, levulinic acid can be derived directly from biomass using an
acid-catalyzed hydrolysis process.
As now synthesized from maleic anhydride, levulinic acid is rather expensive to produce, limiting its use to low-volume applications such as fragrances and food additives. For the biobased version to take off, companies will have to produce it at low cost and work with customers to identify new uses, says Adrian Higson, lead consultant on biobased products at the U.K.’s National Non-Food Crops Centre.
Those firms could look to U.S.-based Segetis, which sells ingredients for personal care and cleaning products based on levulinic ketals. The company operates a biobased levulinic acid pilot plant in Minnesota.
Reaction Chemistry: Study reveals mechanism theoretical work didn’t predict
|
MOUNTAINOUS DARTBOARD This map shows reaction energies calculated for the MBH reaction by theoretical studies (each represented by a set of distinctly colored bars). Conclusive experimental data is also shown (solid black line). Energies are in kcal/mol from ground state (dashed black line). |
Chemists often use computational methods to predict reaction pathways and energies, but some researchers question their usefulness because the models sometimes produce highly variable, head-scratching results. Now, a detailed case study of a multistep organic reaction attacks the utility of computer modeling of that reaction in an unusually blunt way.
The authors conclude that the reaction mechanism is a simple one that undergraduates could guess and that a complex mechanism predicted by years of computational studies is “not even wrong”—so flawed and off-base that calling it incorrect is too kind.
The study by R. Erik Plata and Daniel A. Singleton of Texas A&M University focuses on the Morita-Baylis-Hillman (MBH) reaction, in which an electron-deficient alkene, a nucleophile catalyst, and an aldehyde react to form an allylic alcohol (J. Am. Chem. Soc. 2015, DOI: 10.1021/ja5111392). The researchers conducted a wide range of experiments to nail down the reaction’s mechanism and energetics conclusively for the first time.
Computational studies on the reaction have been “arguably more misleading than enlightening,” they conclude. “It is not clear to us that any reliably accurate information that was not already apparent from experiment could have been garnered from calculations.” The most notable theoretical prediction was that the reaction involves a complex “proton-shuttle” pathway, but experiments find the mechanism to be a simple acid-base process.
Experimental findings are often used to tweak theoretical predictions. In the absence of those tweaks, MBH reaction calculations “could have made an exceptional diversity of predictions, many of which would have been absurd,” Plata and Singleton write. They believe that’s what happened in the case of the proton-shuttle prediction.
Chemical theoretician Kendall N. Houk of UCLA says the paper “is full of profound insights, including one that astute computational chemists are all familiar with”—that they cannot currently use standard computational methods to predict some features of solution reactions. Theory, he says, “is still not capable, and may never be capable, of predicting what happens when many chemicals, four in this case, are mixed in solution.”
The study’s harsh verdict on theory is based only on an analysis of the MBH reaction. Nevertheless, theoretical “studies of complex multimolecular polar reactions in solution should be undertaken and interpreted only with extreme care,” the paper suggests. (See also C&EN, Aug. 15, 2011, page 36.)
Experimental chemist Tyler McQuade of Florida State University says the study “provides a call to arms for our community.” Experimentalists and theoreticians, he says, “need to continue developing methods and testing their veracity with a skeptical eye.”
Organic Chemistry: Chemists bring data-intensive approach to bear on chiral anion catalysts
By combining modern data analysis techniques with classical physical organic and computational chemistry, chemists have developed a way to pin down the mechanism by which a chiral anion catalyst generates certain enantiomers. The method could help chemists rationally design more effective catalysts.
By figuring out a reaction’s mechanism—the precise way the reactants come together to form products—chemists can learn how to tweak that transformation to improve upon it, by boosting the yield, for example. But mechanisms can be complicated, particularly those of enantioselective catalysts in which myriad attractive and repulsive nonbonding forces are at work.
To get a better handle on what was happening in their reaction flasks, University of California, Berkeley, chemists F. Dean Toste and Andrew J. Neel teamed up with University of Utah chemists Matthew S. Sigman and Anat Milo. Together, they took a data-intensive look at an intramolecular dehydrogenative C–N coupling reaction that’s catalyzed by chiral phosphoric acid derivatives (Science 2015, DOI: 10.1126/science.1261043).
Neel performed dozens of permutations of the reaction, tweaking both catalyst and substrate, and then shared his data with Milo, who applied modern data analysis techniques to them. “What they came up with,” Sigman says, “was a model to describe what look like very interesting interactions between substituents on both the catalyst and the substrate.” On the basis of that information, the chemists designed new catalysts, ultimately predicting how they would behave.
“You’ve got all sorts of stuff going on in this reaction, which makes it difficult to figure out with classic kinetics,” Toste says. “But here’s an approach where you take every bit of data you’ve got and you build a model based on physical parameters and combine that with classical physical organic chemistry. Then you’ve got something that’s really powerful that I think anybody could use.”
Steven E. Wheeler, a computational chemistry expert at Texas A&M University, says, “Toste and Sigman have shown that a data-driven approach to mechanistic analyses can complement traditional tools of physical organic chemistry, providing a key step toward a future in which big data is used to design small catalysts.”
Catalytic reactions produce simple organics common in prebiotic chemistry
An iron sulfide mineral that forms in deep sea hydrothermal vents can convert CO2 and hydrogen to small bioorganic molecule precursors such as methanol and formic, acetic, and pyruvic acid (Chem. Commun. 2015, DOI: 10.1039/C5CC02078F). The discovery, by Nora H. de Leeuw of University College London and colleagues, provides a potential lead for developing environmentally friendly catalytic syntheses of plastics and fuels. It also lends credence to the theory that prebiotic chemistry flourished in the mineral- and carbon-rich alkaline environment that typifies some hydrothermal vents. Scientists have known that this mineral, greigite (Fe3S4), resembles the ferredoxin center of the CO dehydrogenase enzyme. In previous studies, researchers showed that greigite can convert CO2 to gaseous CH4 and CO. However, to serve as prebiotic precursors, small organics must be in solution. So the team performed experiments at various pH values and found that an alkaline environment was key to producing methanol and the other small organics that are solution-based at atmospheric pressure and room temperature. And by using computational methods, the group explained the dependence of the methanol and formic acid formation mechanisms on alkaline conditions.
Sustainability: Oxygen vacancies in BiOBr layered catalyst help split nitrogen and water to reduce energy demand for making ammonia
A research team in China has invented a light-harvesting layered semiconductor nanosheet that could one day significantly reduce the energy required for chemically reducing nitrogen to ammonia. Converting N2 to NH3 via the iron-catalyzed Haber-Bosch process is one of the most important industrial chemical reactions. But splitting N2 and preparing hydrogen via steam reforming of methane at high temperature and pressure make it one of the most energy-intensive processes. Lizhi Zhang of Central China Normal University and coworkers designed a layered BiOBr catalyst with oxygen vacancies that is ideal for binding N2 molecules. When the researchers shine visible light on the nanosheet surface, the semiconductor generates electrons to reduce adsorbed N2 while at the same time it oxidizes water solvent molecules to generate H+ and O2. Overall, the process couples nitrogen and hydrogen to make NH3 at room temperature and atmospheric pressure with better efficiency than previously reported semiconductor systems (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b03105). “Although photocatalytic reduction is unlikely to replace the Haber-Bosch process at present”, the researchers write, “this study might open up a new vista”.
ChemCatChem
Nanostructured Metallic Electrocatalysts for Carbon Dioxide Reduction
Qi Lu, Jonathan Rosen, Feng Jiao
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/cctc.2014026690
Advanced Synthesis & Catalysis
N-Imide Ylide-Based Reactions: C–H Functionalization, Nucleophilic Addition and Cycloaddition
Guanyinsheng Qiu, Yunyan Kuang, Jie Wu
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/adsc.201400631
ChemElectroChem
Redox-Active Functionalized Graphene Nanoribbons as Electrode Material for Li-Ion Batteries
Klemen Pirnat, Jan Bitenc, Ivan Jerman, Robert Dominko, Bostjan Genorio
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/celc.201402234
Angewandte Chemie International Edition
Fast Prediction of Adsorption Properties for Platinum Nanocatalysts with
Generalized Coordination Numbers
Federico Calle-Vallejo, José I. Martínez, Juan M. García-Lastra, Philippe Sautet, David Loffreda
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/anie.201402958
Chemistry – A European Journal
Palladium-Catalyzed α-Arylation of Arylketones at Low Catalyst Loadings
Enrico Marelli, Martin Corpet, Sian R. Davies, Steven P. Nolan
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/chem.201404900
Macromolecular Chemistry and Physics
Assessing Catalytic Activities through Modeling Net Charges of Iron Complex recatalysts
Wenhong Yang, Yan Chen and Wen-Hua Sun
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/macp.201400141
Energy Technology
Heterogeneously-Catalyzed Hydrogenation of Carbon Dioxide to Methane using RuNi Bimetallic Catalysts
Felix Lange, Udo Armbruster, Andreas Martin
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/ente.201402113
ChemPhysChem
Forced Folding of a Disordered Protein Accesses an Alternative Folding
Landscape
Mahdi Muhammad Moosa, Allan Chris M. Ferreon, Ashok A. Deniz
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/cphc.201402661
ChemPlusChem
ZnO Rhombic Sheets of Highly Crystalline Particles and Their Composite
with Ag2O toward Efficient Photocatalysis
Xiaobin Dong, Ping Yang, Junpeng Wang, Baibiao Huang
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/cplu.201402182
Chemistry – An Asian Journal
Integration of [(Co(bpy)3]2+ Electron
Mediator with Heterogeneous Photocatalysts for CO2 Conversion
Jinliang Lin, Yidong Hou, Yun Zheng, Xinchen Wang
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/asia.201402303
The Chemical Record
Asymmetric Synthesis of Agrochemically Attractive Trifluoromethylated
Dihydroazoles and Related Compounds under Organocatalysis
Hiroyuki Kawai, Norio Shibata
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/tcr.201402023
Applied Organometallic Chemistry
Synthesis of diaryl thioethers from aryl halides and potassium
thiocyanate
Hajipour A. R., Pourkaveh R., Karimi H.
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/aoc<.3230
Journal of Physical Organic Chemistry
Fractional distribution of graphene oxide and its potential as an efficient and reusable solid catalyst for esterification reactions
Mungse H. P., Bhakuni N., Tripathi D., Sharma O. P., Sain B., Khatri O. P.
http://onlinelibrary.wiley.com/enhanced/doi/10.1002/poc.3375