

Валентин Николаевич – выпускник Московского физико-технического института. В 1977 г. был приглашен в Институт катализа Сибирского отделения АН СССР. В 1985 г. возглавил лабораторию каталитических методов преобразования солнечной энергии, а в 1995 г. стал директором Института катализа им. Г.К. Борескова СО РАН.
Основными направлениями научной деятельности В.Н. Пармона являются разработка и исследование катализаторов и каталитических процессов, в том числе для преобразования и аккумулирования различных видов энергии, катализ и фотокатализ в природе и в использовании возобновляемых и нетрадиционных энергоресурсов, а также выяснение роли абиогенных каталитических и фотокаталитических процессов в формировании состава атмосферы Земли и зарождении биосферы.
В.Н. Пармоном разработаны научные основы фотокаталитических методов преобразования солнечной энергии в химическую (разложение воды на водород и кислород в искусственных системах).
В.Н. Пармоном создано новое научное направление – радиационно-термический катализ. Под его руководством сконструированы и испытаны не имеющие мировых аналогов солнечные каталитические реакторы, остающиеся сегодня самыми эффективными из известных (эффективность преобразования солнечной энергии достигает 43% при полезной мощности 2 кВт).
В.Н. Пармоном разработан принципиально новый подход к прямому преобразованию ионизирующего излучения в энергию химических топлив. В результате предложен и испытан в лабораторных условиях энергозапасающий и энергопреобразующий процесс “ИКАР”, который может быть эффективно использован для решения проблем ядерной и термоядерной энергетики будущего. Впервые созданы и испытаны не имеющие аналогов катализаторы на основе оксидов урана, комбинирующие функции ядерного топлива и катализатора для запасания химической энергии.
Под руководством В.Н. Пармона разработаны принципиально новые центробежные реакторы для термоударной обработки порошков твердых субстратов и отработана технология получения с помощью этих реакторов порошков активной окиси алюминия. Данная технология с 2009 года используется для промышленного производства новейшего поколения мелкосферических катализаторов дегидрирования легких алканов в олефины, уже нашедших промышленное применение для дегидрирования изобутана в изобутилен в кипящем слое катализатора на предприятии “Тобольск-Нефтехим” нефтехимической компании ОАО “СИБУР-холдинг”. Экономический эффект от использования нового катализатора превысил 200 млн. руб.
Под руководством В.Н. Пармона в 2003–2006 годах разработаны и промышленно внедрены катализаторы нового поколения для производства моторных топлив.
В последнее десятилетие под руководством В.Н. Пармона в кооперации с зарубежными партнёрами успешно ведутся работы по новым перспективным направлениям энергетики и транспорта (получение высококачественных топлив из возобновляемого растительного сырья, создание компактных генераторов водорода и др.).
В.Н. Пармон – член Президиумов РАН и СО РАН, председатель Объединенного ученого совета по химическим наукам СО РАН, председатель Научного совета по катализу ОХНМ РАН; член Консультативного научного Совета Фонда “Сколково”.
В.Н. Пармон – автор и соавтор более 600 научных работ, 6 монографий, 33 обзоров, 5 учебников для вузов, обладатель более 100 авторских свидетельств и патентов.
В течение 25 лет Валентин Николаевич преподает в Новосибирском Государственном университете, являясь профессором кафедры физической химии факультета естественных наук. Под его руководством подготовлены 5 докторов наук и 17 кандидатов наук.
В.Н. Пармон – бессменный главный редактор журнала “Катализ в промышленности” и Каталитического бюллетеня, а также редактор журнала “Химия в России” (РХО), член редколлегий журналов “Успехи химии”, “Кинетика и катализ”, “Журнала физической химии” (РАН), ряда международных журналов, Российский национальный представитель в EFCATS и IACS, член Президиума РХО им. Д.И. Менделеева.
Заслуги В.Н. Пармона отмечены высокими государственными наградами, среди которых Орден Почета (1999 г.), Орден “За заслуги перед Отечеством” IV степени (2007 г.), Медаль Франциска Скорины – за личный вклад в научное сотрудничество и разработку совместных белорусско-российских наукоемких производств (2009 г.), Государственная премия РФ 2009 года в области науки и технологий.
Научный совет по катализу ОХНМ РАН и редакция
Каталитического бюллетеня сердечно поздравляют
Валентина Николаевича с юбилеем, желают ему крепкого здоровья и новых творческих достижений!
 | 55-летний юбилей |
История и деятельность Института катализа им. Г.К. Борескова Сибирского отделения РАН – крупнейшего химического института Российской академии наук к востоку от Урала и самого крупного мирового исследовательского центра, специализированного в области катализа, – неразрывно связаны с развитием науки и химической промышленности СССР, а нынче – России.
Директором-организатором Института катализа СО АН в 1958 году стал заведующий лабораторией технического катализа физико-химического института им. Л.Я. Карпова Минхимпрома СССР член-корр. Г.К. Боресков. В состав первой дирекции Института катализа вошли три человека: директор – Г.К. Боресков и два его заместителя по научной работе – М.Г. Слинько и Р.А Буянов. Численность сотрудников Института на конец 1959 года – 72 человека.
Кадровый костяк Института в начальный период составили сотрудники Научно-исследовательского физико-химического института им. Л.Я. Карпова, Института физической химии АН СССР, выпускники московских и ленинградских вузов.
В соответствии с Уставом Института катализа СО РАН его основной задачей является выполнение фундаментальных научных исследований и прикладных разработок по следующим направлениям:
Становление и развитие Института катализа неразрывно связано с деятельностью его директоров.
 Академик Г.К. Боресков – основатель Института катализа и его первый директор (с 1958 по 1984 гг.), выдающийся ученый-химик, инженер, организатор науки, замечательный педагог. Работы Г.К. Борескова в области катализа, химической кинетики и химической технологии получили мировое признание. В 1991 году институту присвоено имя Г.К. Борескова.
Академик Г.К. Боресков – основатель Института катализа и его первый директор (с 1958 по 1984 гг.), выдающийся ученый-химик, инженер, организатор науки, замечательный педагог. Работы Г.К. Борескова в области катализа, химической кинетики и химической технологии получили мировое признание. В 1991 году институту присвоено имя Г.К. Борескова.
 Академик К.И. Замараев – выдающийся физико-химик, известный в России и за рубежом исследованиями механизмов каталитических реакций на молекулярном уровне, прекрасный лектор и педагог, великолепный организатор. К.И. Замараев возглавлял Институт катализа с 1984 по 1995 годы.
Академик К.И. Замараев – выдающийся физико-химик, известный в России и за рубежом исследованиями механизмов каталитических реакций на молекулярном уровне, прекрасный лектор и педагог, великолепный организатор. К.И. Замараев возглавлял Институт катализа с 1984 по 1995 годы.
 Академик В.Н. Пармон – директор Института с 1995 года. В.Н. Пармон является известным ученым в области катализа и фотокатализа, химической кинетики в конденсированных фазах, термодинамики неравновесных процессов, каталитических методов глубокой переработки углеводородного сырья и использования возобновляемых ресурсов. Под руководством академика В.Н. Пармона в 2000-2010 гг. Институт катализа СО РАН стал в России лидером инновационной деятельности в области химической промышленности и природоохранных технологий.
Академик В.Н. Пармон – директор Института с 1995 года. В.Н. Пармон является известным ученым в области катализа и фотокатализа, химической кинетики в конденсированных фазах, термодинамики неравновесных процессов, каталитических методов глубокой переработки углеводородного сырья и использования возобновляемых ресурсов. Под руководством академика В.Н. Пармона в 2000-2010 гг. Институт катализа СО РАН стал в России лидером инновационной деятельности в области химической промышленности и природоохранных технологий.
В настоящее время Институт катализа – уникальное объединение специалистов в различных областях науки и технологии, способных решать любые задачи в области катализа: от фундаментальных проблем до дизайна промышленных катализаторов и процессов. В Институте сейчас работает около 1000 человек. Среди 400 научных сотрудников Института более 70 докторов и 200 кандидатов наук. Более трети научных сотрудников – молодые ученые.
За пять лет, прошедших с 50-летнего юбилея Института катализа СО РАН (2008 г.), получили дальнейшее развитие современные методы исследования строения и свойств катализаторов в режиме in situ. Благодаря этим исследованиям были разработаны новые подходы для создания катализаторов глубокого и селективного окисления. В качестве только одного примера можно упомянуть создание нового поколения катализаторов очистки газов от оксида углерода при комнатных температурах. Управление свойствами поверхности носителей и дисперсностью активного компонента (палладия) позволило снизить содержание драгметалла в катализаторе в 2,5-3,0 раза по сравнению с используемыми ныне катализаторами.
Дальнейшее развитие получили работы в области промышленного катализа.
В 2009 году совместно с РНЦ «Прикладная химия» разработан современный отечественный катализатор синтеза озонобезопасных хладонов и технология его производства. Опытно-промышленные испытания вновь разработанного катализатора на ОАО «Галоген» (г. Пермь) продемонстрировали его высокие потребительские свойства: снижение энергозатрат и расходных коэффициентов по сырью, увеличение производительности существующих реакторов в 3-5 раз.
В 2011 году завершены исследования, освоена технология производства, наработана опытно-промышленная партия и начата эксплуатация медьсодержащих катализаторов синтеза монометиланилина на ОАО «Крата» (г. Тамбов). Разработанный процесс синтеза монометиланилина позволил увеличить срок непрерывной работы катализатора практически в 3 раза.
В 2010-2011 годах большой объем исследований был связан с разработкой технологических основ применения палладийсодержащих катализаторов в гидрировании растительных масел. Наработанные опытно-промышленные партии палладийсодержащих катализаторов были использованы для производства пищевых саломасов на ООО «ЭФКО Пищевые Ингредиенты» (г. Алексеевка). Общий объем произведенной на основе палладийсодержащих катализаторов продукции составил около 1000 тонн.
В 2010-2012 годах получило широкое развитие строительство высокоэффективных экологически чистых угольных котельных на основе каталитического сжигания топлива. Работа таких котельных основана на сжигании твердого топлива в кипящем слое дисперсного катализатора. За счет увеличения степени выгорания топлив и снижения теплопотерь расход топлива сокращается на 25-30%. Существенным преимуществом котельных является их экологическая безопасность и снижение выбросов загрязняющих веществ (оксиды азота, серы и др.) в атмосферу более чем в 10 раз. К настоящему времени в России введено в эксплуатацию пять таких котельных суммарной мощностью около 20 МВт.
Разработанные совместно с ООО «НПК «Синтез» катализаторы дегидрирования легких углеводородов в кипящем слое получили в 2010-2013 гг. широкое распространение. В настоящее время объем производства таких катализаторов на ООО «НПК «Синтез» превышает 2-2,5 тыс. тонн/год, и эти катализаторы стали базовыми для производств мономеров синтетического каучука на ОАО «СИБУР Холдинг».
Тесное сотрудничество с ОАО «Газпром» и ООО «Новомичуринский катализаторный завод» позволило разработать новую технологию производства шарикового высокоактивного катализатора для процесса Клауса. По сравнению с известными российскими и импортными катализаторами активность вновь разработанного катализатора выше в 1,5-2,0 раза, что позволяет прогнозировать более длительный срок его промышленной эксплуатации. В 2013 году первая промышленная партия катализатора в объеме 100 тонн наработана на ООО «Новомичуринский катализаторный завод» и введена в эксплуатацию на газоперерабатывающем заводе ОАО «Газпромдобыча Оренбург».
Институт катализа СО РАН в ближайшее время планирует расширить исследования в области катализаторов нефтепереработки и, прежде всего, катализаторов для гидрогенизационных процессов. Накопленный потенциал позволяет уже в ближайшие годы перейти к использованию вновь разработанных катализаторов в нефтеперерабатывающей промышленности.
Институт катализа СО РАН вступает в дальнейшую жизнь с серьезными научными планами и активной позицией при сотрудничестве с российской промышленностью.
1-6 июля 2012 г.
Мюнхен (Германия)
C 1 по 6 июля 2012 года в г. Мюнхене (Германия) прошел 15-й Международный Конгресс по катализу. Для его проведения использовался современный Конгресс-центр в пригороде Мюнхена, который легко вместил около 2 тысяч участников из 51 страны мира.

 |
Конгресс-центр расположен на окраине Мюнхена в быстро развивающейся бизнес-зоне на территории бывшего городского аэропорта, от которого сохранилась только старая диспетчерская башня. Эффективному проведению конгрессов и конференций, несомненно, способствует огромный пруд перед Конгресс-центром, рядом с которым хорошо размышлять о проблемах науки и технологии, глядя на лилии или на разгуливающих по берегу гусей. |
 |
Работу по организации Конгресса проводило Немецкое Химическое Общество (Дехема), а Организационный Комитет возглавил профессор Йоханнес Лерхер из Технического Университета Мюнхена, главный редактор Journal of Catalysis, который открыл заседания Конгресса.

Тематика Конгресса распределялась по трем основным и достаточно традиционным темам:
A. Механизм – установление природы действия катализаторов и детальных маршрутов протекания каталитических реакций.
B. Материалы – дизайн, синтез и характеризация катализаторов.
C. Процессы – новые подходы к реализации каталитической трансформации веществ.
По областям применения тематика Конгресса структурировалась по четырем направлениям:
Программа Конгресса включала 6 пленарных лекций, прочитанных ведущими специалистами в области катализа, в том числе:
Две лекции были сделаны ведущими учеными по случаю вручения им наград:
Кроме того, в рамках соответствующих сессий Конгресса были прочитаны 14 ключевых лекций:
На ряде секций лекции были прочитаны приглашенными специалистами, в том числе шесть лекций на междисциплинарном совещании по органометаллическому катализу:
две лекции – на междисциплинарном совещании по органокатализу/биокатализу:
и одна лекция – на междисциплинарном совещании по фотокатализу:
Далее при обсуждении работы Конгресса при ссылке на такие лекции будет в основном указываться только имя лектора.
Следует особо отметить впервые предложенную в рамках данного Конгресса по катализу (впрочем, уже апробированную на конференциях Дехема) весьма интересную форму представления научных материалов в виде кратких устных пятиминутных докладов, сгруппированных в 36 постерных Симпозиумов по всей тематике Конгресса с продолжительностью каждого симпозиума всего 1 час 40 минут. Не утомляющие долгими введениями и выводами, флэш-доклады практически не уступали по информативности традиционным полновесным устным докладам и, вместе с тем, полностью фокусировали внимание слушателей. Небольшие помещения, где проводились данные симпозиумы, с трудом вмещали всех желающих, царила особая непринужденная научная атмосфера, задаваемые вопросы носили конкретный характер и зачастую спонтанно переходили в неформальные бурные флэш-дискуссии собравшихся специалистов в кулуарах.
Другой особенностью работы с постерными докладами Конгресса, помимо двух традиционных постерных сессий и обсуждения со стоящими у стендов авторами, была возможность загрузки любого постера на огромные экраны, размещенные в Конгресс-центре, и их детального изучения во все дни работы Конгресса.
В области нефтепереработки ключевая лекция была прочитана проф. Бао (X. Bao) из Института химической физики, Далян, Китай, “Уникальная каталитическая химия в наноограниченных системах”. В 10 устных докладах были рассмотрены различные аспекты гидроочистки, изомеризации и гидрокрекинга. Интересные стендовые доклады были представлены сотрудниками ИППУ СО РАН (г. Омск) – одной из самых многочисленных российских делегаций в составе тринадцати человек во главе с директором В.А. Лихолобовым.

В.А. Лихолобов в рамках своего доклада представил новые подходы к синтезу платиновых катализаторов реакций превращения углеводородов. Работа О.Б. Бельской была посвящена особенностям приготовления нанесенных на гидротальциты платиновых катализаторов реакции дегидрирования пропана. М.О. Казаков и Е.Д. Федорова представили результаты своих исследований, посвященных проблеме получения экологически чистых моторных топлив с пониженным содержанием бензола. Доклад Л.Ф. Сайфулиной был посвящен актуальной проблеме превращения газового сырья в компоненты жидких моторных топлив в реакции олигомеризации этилена, а работа А.А. Волкова касалась вопросов математического моделирования данного процесса. А.В. Василевич сделала доклад об особенностях приготовления нанесенных никель-молибденовых катализаторов для процесса гидроочистки тяжелых нефтяных фракций. В докладе Е.А. Булучевского была затронута проблема превращения этилена в пропилен в одну технологическую стадию на никель-рениевом катализаторе. Работы О.О. Мироненко и Ю.С. Котолевич были посвящены приготовлению нанесенных палладиевых и серебряных катализаторов методами горения (ПСТ и ИПТ) для процессов гидрирования и окисления. В своем докладе Р.М. Мироненко с помощью современных физико-химических методов исследования рассмотрел особенности влияния поверхности оксида алюминия на формирование платиновых активных центров.
Традиционно для последнего времени, на 15-м Международном Конгрессе по Катализу были широко представлены доклады, связанные с переработкой компонентов биомассы в ценные химические соединения и альтернативное топливо. Так, в секции “Превращение биомассы в топливо” (“Biomass conversion to fuels”) были представлены 1 ключевой, 15 устных и более 100 стендовых докладов. В. Ордомский (TU Eindhoven, Голландия) сделал интересный доклад о дегидратации фруктозы в 5-гидрокси-метилфурфурол в присутствии структурных гетерогенных катализаторов. В докладе T. Oyama (The University of Tokyo, Япония) представлены результаты детального изучения механизма гидродеоксигенирования в присутствии фосфидов переходных металлов. Интересный доклад был сделан M. Renz (Polytechnic University of Valencia, Испания) из группы A. Corma о получении биодизеля второго поколения из ключевых соединений, производных гемицеллюлозы и целлюлозы. Ряд докладов был посвящен разработке новых подходов к каталитической переработке лигнинов. C. Zhao (TU Munchen, Garching, Германия) рассказал о получении газолина, керосина и дизеля путем гидродеоксигенирования лигнинов, T. Vom Stein (RWTH Aachen University, Германия) рассмотрел подходы к селективному разрыву связей лигнина на молекулярном уровне, R. Rinaldi (MPI for Coal Research, Mulheim, Германия) проанализировал влияние растворителей на гидрогенолиз лигнина на Ni Ренея.
В секции “Ключевые химические соединения и соединения специального назначения из возобновляемого сырья” ("Platform and specialty chemicals from renewables") были представлены 1 ключевой, 15 устных и более 100 стендовых докладов. Профессор Роберт Дэвис из Университета Виргинии (Шарлоттсвилл, США) выступил с ключевой лекцией “Критическая роль OH в селективном окислении или гидрогенолизе молекул производных биомассы в присутствии нанесенных металлических катализаторов” (см. выше). Замечательный доклад был прочитан профессором Д.Ю. Мурзиным (Университет Або Академи, Финляндия). Он был посвящен совмещенному процессу гидролиза гемицеллюлоз и гидрированию получающихся сахаров в полиолы с использованием металл-нанесенных катализаторов. Профессор A. Fukuoka (Hokkaido University, Япония) рассказал об особенностях превращения лигноцеллюлозы в ключевые химические соединения в присутствии нанесенных на углерод металлических катализаторов. В докладе P. Dapsens (ETH, Швейцария) были продемонстрированы интересные возможности иерархических цеолитных катализаторов для превращения компонентов биомассы, а в докладе Y. Nakagawa (Tohoku University, Япония) показана высокая активность и селективность иридий-рениевых катализаторов для селективного гидрогенолиза глицерина в 1,3-пропандиол.
Постерный симпозиум 37 (сопредседатели проф. R. Palkovits (RWTH Aachen, Германия) и проф. Д.Ю. Мурзин (Аbo Akademi University, Финляндия) был специально посвящен актуальному вопросу переработки древесины – деполимеризации лигнина и превращению модельных лигниновых соединений. В рамках данной секции были заслушаны различные доклады, часть из которых была посвящена каталитическому синтезу из лигнинов химических соединений и водорода, например A.L. Jongerius (University of Utrecht, Голландия) или гидродеоксигенированию, например доклад H. Ohta (Hokkaido University, Япония). О.А. Симакова (Аbo Akademi University, Финляндия) выступила с докладом по селективному окислению на золоте сложных соединений лигнанов, получаемых из древесной биомассы. Доклад вызвал особый интерес, поскольку применение золотосодержащих катализаторов для превращения сложных многофункциональных органических молекул является само по себе малоизученной областью. Этой проблеме был посвящен постерный симпозиум 32 “Контролируемый синтез золотых частиц и их сплавов” (“Tailored Au and Au alloy particles”) под председательством C. Louis, (Universite Pierre et Marie Curie, Франция). Доклад профессора Е.В. Гусевской (Universidade Federal de Minas Gerais, Бразилия) был посвящен селективному аэробному окислению спиртов в присутствии золотых наночастиц на оксиде магния. В докладе C. Chmelik (University of Stuttgart, Германия) было продемонстрировано применение микро ИК для in-situ исследований химических реакций в нанопорах катализатора. Профессор А.В. Симаков (Centro de Nanociencias y Nanotecnologia – UNAM, Мексика) представил доклад о возможностях метода UV-Vis-mass in-situ для исследования процесса формирования золотых наночастиц на поверхности носителя и их взаимодействия с реакционной средой.
Важно отметить, что тема синтеза золотых частиц и их применения в катализе в настоящее время приобрела особую популярность, что было отражено в лекции проф. G. Hutchings по случаю присуждения ему премии за работы в данной области (см. выше).
Вопросы трансформации биотоплив в синтез-газ были также рассмотрены на секции “Получение и конверсия синтез-газа”. От имени международного российско-ирландско-французского коллектива авторов, Н.В. Мезенцевой (ИК СО РАН, Россия) был сделан устный доклад “Каталитическая трансформация биотоплив в синтез-газ и водород на блочных катализаторах” (один из 5 устных докладов от Института катализа СО РАН), который получил награду Международного Конгресса для молодых ученых.
 На предыдущем Конгрессе по катализу в Сеуле Н. Мезенцева получила награду Конгресса за стендовый доклад по данной тематике.
На предыдущем Конгрессе по катализу в Сеуле Н. Мезенцева получила награду Конгресса за стендовый доклад по данной тематике.
Необходимо отметить, что еще один молодой ученый от Института катализа – М. Симонов (группа И. Симаковой) также получил награду 15-го Конгресса за стендовый доклад “Design of highly selective propylene glycol synthesis via hydrogenolysis of alkyl lactates over Cu/SiO2”.
На данной секции также были представлены доклады по паровой конверсии этанола, метанола, селективному окислению и риформингу углеводородов в синтез-газ, паровой конверсии СО, а также по синтезу Фишера-Тропша и метанола. Проблемы кинетики и механизма селективного окисления, паровой и углекислотной конверсии углеводородов на катализаторах разного типа и факторы, определяющие их стабильность к зауглероживанию, были также рассмотрены на постерном симпозиуме 9 “Риформинг углеводородов в синтез-газ” (сопредседатели проф. В. Садыков, Институт катализа им. Борескова, и проф. Ф. Рибейро, Университет Пердью, Вест Лафайет, США). В докладе российско-французского коллектива (Институты катализа в Новосибирске и Лионе) “Mechanism of CH4 dry reforming on oxides with high oxygen mobility promoted by Pt, Ru, Ni and Ni-Ru”, представленном В. Садыковым, было впервые показано, что на катализаторах данного типа механизм описывается простой стадийной схемой с активацией метана на кластерах металлов, диссоциацией СО2 на восстановленных центрах поверхности носителя – сложного оксида со структурой перовскита или флюорита – с их реокислением и образованием СО, а сопряжение стадий обеспечивается быстрой диффузией кислорода с центров носителя к границе раздела металл-оксид. Проблемы углекислотной конверсии метана на модифицированных катализаторах Ni/Al2O3 были также рассмотрены в докладе С. Сенгупта (Канпур, Индия) на секции “Catalysis in CO2 capture, sequestration and utilization”. Проблемы паровой конверсии спиртов были рассмотрены на постерном симпозиуме 41 (сопредседатели С. Чин, Университет Торонто, Канада, и К. Сешан, Университет Твента, Голландия).
В секции "Междисциплинарный семинар по фотокатализу" были представлены 1 ключевой, 15 устных и более 100 стендовых докладов. Кроме того, пленарная лекция проф. Kazunari Domen (Япония) на открытии Конгресса была посвящена фотокаталитическому разложению воды на кислород и водород. Профессор Jean-Marie Herrmann выступил с лекцией об историческом развитии фотокатализа в течение последних 50 лет. Были отмечены основные направления развития фотокатализа в 20 веке и современные перспективы развития фотокаталитических процессов. В лекции профессора M. Bowker (Великобритания) “Фотокаталитическое выделение водорода: роль активных центров” было рассмотрено влияние нанесения различных благородных металлов на поверхность диоксида титана на активность в реакции фотокаталитического образования водорода. Ключевая лекция профессора Can Li (Китай) была посвящена роли со-катализаторов и гетеропереходов в фотокаталитическом преобразовании солнечной энергии. Была показана перспективность использования многофазных полупроводниковых систем, чувствительных к видимому свету. Доклады K. Maeda и Q. Jia из Японии были посвящены Z-схеме фотокаталитического разложения воды. Обсуждались фотокатализаторы и доноры электронов для проведения реакции выделения водорода по данной схеме.
Приглашенная лекция профессора S. Malato (Испания) и доклад профессора C. Pulgarin (Швейцария) были посвящены фотокаталитической очистке от вредных органических веществ и дезактивации микроорганизмов в воде с использованием фотокатализа. Темой лекции профессора D. Uner (Турция) была термодинамика фотокаталитического восстановления углекислого газа. Было показано, что фотокатализ – один из термодинамически разрешенных путей утилизации CO2. W. Wark (Германия), J. Zhao (Китай), V. Jovic (Новая Зеландия) в своих докладах рассказали об особенностях формирования наноразмерного диоксида титана для различных реакций – полного и парциального окисления, фотообразования водорода. Екатерина Козлова (Институт катализа СО РАН) представила устный доклад по теме “Фотокаталитическое выделение водорода из водных растворов глицерина под видимым светом”.

Екатерина Козлова (справа) после доклада с коллегами из Института катализа, ныне работающими за рубежом:
Ольга Овсицер (Берлин, Германия) и Сергей Белошапкин (Университет Лимерика, Ирландия).
Среди более традиционных направлений достаточно широко были представлены работы по селективному окислению (секция по селективному окислению, а также постерные симпозиумы по окислительному дегидрированию, селективному окислению спиртов, селективному окислению ВТХ и гетерополикислотам). Катализ в топливных элементах также был представлен как на секции устных докладов, так и на постерном симпозиуме, что позволило рассмотреть проблемы катализа в полимерных и твердооксидных топливных элементах. Для твердооксидных топливных элементов показано, что применение нанокомпозитных материалов на основе допированного диоксида церия или церий-циркониевых смешанных оксидов позволяет создать высокоэффективные и устойчивые к зауглероживанию аноды для топливных элементов, работающих на прямом окислении метана или этанола (доклады М. Боаро и др., Университет Удине, Италия; А. Мартинес-Ариас и др., Мадрид, Испания).
Проблемы очистки выбросов автотранспорта от СО, углеводородов и оксидов азота были представлены как на секции устных докладов “Выбросы автотранспорта” с ключевой лекцией Р. Фаррауто (США), так и на секции “Очистка выбросов” с ключевой лекцией В. Бухтиярова (см. выше и ниже). Среди перспективных типов каталитических систем большое внимание уделяется как массивным, так и нанесенным смешанным оксидам на основе циркония-церия, промотированным металлами и оксидами переходных металлов. Были представлены интересные результаты по применению современных спектральных и структурных методов in situ в сочетании с теоретическими методами для изучения механизма реакций окисления СО и углеводородов, селективного восстановления оксидов азота, например, в докладах Р. Берча и др. (Квинс Университет, Белфаст), “The development of Short Time on Stream (STOS) SSITKA to identify true reaction intermediates in automotive catalysis”, Г. Кьярелло и др. (Швейцария/Франция) “Red-ox dynamics of Al2O3 and Ce1-xZrxO2 supported Pd studied by time-resolved modulation excitation hard X-ray diffraction”.
На секции по промышленному применению были в основном представлены доклады от крупных химических корпораций – БАСФ, Байер, Дженерал Моторс, Джонсон Мэтью, Сасол и др., в которых был обобщен их опыт по созданию катализаторов для крупномасштабных процессов получения хлора, синтеза Фишера-Тропша, получению олефинов из метанола и т.д.
На секции “From mechanistic insides to advances in reactor technology” были рассмотрены аспекты дизайна реакторов (включая микроканальные реакторы) для различных типов каталитических реакций на основе детального изучения кинетики и процессов тепло- и массопереноса.
Следует отметить полное отсутствие в программе Конгресса докладов по каталитическому сжиганию топлив для энергетического применения, что говорит о сворачивании некогда популярного направления. В то же время было представлено несколько постеров по каталитическому сжиганию хлорорганики и ароматики для экологического применения.
Несмотря на практическую направленность Конгресса, ряд лекций был посвящен фундаментальным исследованиям модельных систем с применением поверхностно-чувствительных методов. Среди наиболее ярких докладов по данному направлению следует выделить пленарный доклад профессора Фройнда из Института Фрица-Хабера (Берлин, Германия), а также ключевую лекцию профессора Бухтиярова из Института катализа (Новосибирск, Россия), сделанную им в соавторстве с профессором Стахеевым из Института органической химии (Москва, Россия).
Профессор Фройнд рассказал о направлении модельных исследований, на протяжении многих лет развиваемом в Институте Фрица-Хабера, в основе которого лежит изучение электронных и каталитических свойств наночастиц переходных металлов и их оксидов, нанесенных на тонкую оксидную пленку на поверхности монокристаллов металлов (Pt(111), NiAl(110) и т.п.). Данные модельные системы дают возможность, с одной стороны, применять широкий ряд современных поверхностно-чувствительных методов, таких как рентгеновская фотоэлектронная спектроскопия (РФЭС), XANES, ИК-спектроскопия, спектроскопия суммарных частот (SFG), сканирующая туннельная микроскопия и т.п., позволяя определять химический состав, морфологию и электронные и адсорбционные свойства нанесенных наночастиц, а с другой стороны, применяя метод температурно-программируемой десорбции или технику молекулярных пучков, определять их каталитические свойства в зависимости от подложки, используемой в качестве носителя, а также размера наночастиц. Преимущества подхода продемонстрированы на примере исследований ряда важных каталитических реакций, в частности селективного окисления метанола на ванадий-цериевых оксидных катализаторах.

Группа участников Конгресса от Института катализа перед входом в Конгресс-центр. В центре – В. Бухтияров (после выступления) и Г. Бухтиярова. А. Степанов (второй справа) с сумкой БАСФ в руках еще только готовится к докладу.
В ключевом докладе профессора Бухтиярова на секции “Mobile source emission” был предложен альтернативный подход проведения модельных исследований, основанный на приготовлении высокодисперсных катализаторов с узким распределением размеров нанесенных наночастиц. Последнее крайне важно, так как позволяет изучать не только влияние взаимодействия металл-носитель на каталитические свойства, но и природу размерного эффекта. В основе данного подхода лежит синтез серии катализаторов, отличающихся средним размером нанесенных наночастиц, их охарактеризование методами просвечивающей электронной микроскопии, EXAFS, XANES, РФЭС и т.п., а также тестирование в проточном реакторе в условиях, максимально приближенных к реальному катализу. Кроме того, проводится in situ исследование с применением методов РФЭС, XANES и масс-спектрометрии, что позволяет построить корреляцию между каталитическими свойствами и электронным и химическим состоянием активного компонента. Возможности данного подхода были продемонстрированы на примере исследования размерного эффекта в реакции окисления метана на катализаторах Pd/Al2O3, а также окисления СО на катализаторах Au/Al2O3.
В области использования ЯМР спектроскопии твердого тела для характеристики химических реакций, протекающих на поверхности гетерогенных катализаторов, на Конгрессе наметилась тенденция к применению этого метода для установления механизмов активации и неокислительного превращения метана в ароматические углеводороды, а также установления детальных механизмов превращения метанола в олефины (этилен, пропилен) на цеолитных катализаторах типа ZSM-5 и кремний алюмофосфатах (типа SAPO-34). А.Г. Степановым (Институт катализа СО РАН) на секции “Катализаторы для получения чистых топлив” был сделан доклад, проливающий свет на пути активации метана в реакции его совместной ароматизации c более высокомолекулярными алканами (С3-С4), а также на направления активации самих С2-С4 алканов в реакции их ароматизации (без участия метана) на Zn и Ga-модифицированных цеолитах типа ZSM-5 или BEA.

В перерывах между заседаниями можно и немного освежиться – соком, кофе и пр.
На этой же секции С. Ксю (S. Xu) из Даляньского Института химической физики (КНР) представил доклад об идентификации методом ЯМР in situ ключевых интермедиатов, обеспечивающих высокую селективность превращения метанола в пропилен на катализаторе SAPO-34. В докладе отчетливо прозвучало, что интермедиатами, обеспечивающими высокую селективность по олефинам, являются алкил-замещенные бензониевые и циклопентадиенильные катионы, деалкилирование которых приводит к образованию олефинов. Аналогичные выводы о роли замещенных бензониевых катионов в селективном получении олефинов из метанола были сделаны в докладе K. Hernelsoet (University of Utrecht, NL) при исследовании аналогичных каталитических систем комбинацией теоретических и in situ спектроскопических (отличных от ЯМР) методов (секция “Получение и конверсия олефинов”).
На секции “From mechanistic insides to advances in reactor technology” от коллектива сотрудников Томографического центра и Института катализа СО РАН К. Ковтуновым был представлен доклад “Heterogeneous hydrogenation reaction mechanism evaluation and NMR imaging of catalytic hydrogenation by using parahydrogen”.
Проблемы применения физических методов к исследованию катализаторов и каталитических реакций были рассмотрены на ряде постерных симпозиумов, таких как 15 “Изображение/локальные пробы для характеризации катализаторов”, 31 “Синхротронные методы для характеризации катализаторов”, 36 “Современная характеризация с использованием ЯМР”, 40 “Методы in situ для охарактеризования катализаторов и реакций” и 43 “Достижения в электронной микроскопии”.
На Конгрессе были также представлены выставки промышленных фирм, производителей оборудования и издательских компаний. Среди них особенно выделился в этот раз Эльзевир, одарявший ученых, публикующихся в его журналах, рубашками с персональными штрих-кодами.
Среди 6 устных докладов, сделанных российскими учеными, только один был представлен сотрудником не из Новосибирска, а из Москвы – И. Ивановой (Химический факультет МГУ им. М.В. Ломоносова) под названием “Hydroalkylation of benzene with acetone over bifunctional solid catalysts” на секции “Transformation of aromatic molecules and heteroatom functionalization”.
Вероятно, Российскому каталитическому обществу и Совету по катализу необходимо проанализировать причины столь незначительного процента устных докладов от России. Заметим, впрочем, что от других стран СНГ их не было вообще. На последнем Европейском Конгрессе по катализу в Глазго в 2011 году, с меньшим числом участников, Россия была представлена 45 участниками (12 от Института катализа) с 9 устными докладами (5 от Института катализа, один из Красноярска, 4 из Москвы). Сравнение с Международном Конгрессом по катализу указывает на усиление негативных тенденций в представлении российской науки по катализу на международных конференциях.
Хотя Российская делегация была относительно немногочисленной, достаточно много российских ученых, работающих за рубежом, также приняли участие в работе Конгресса. Так, например, непременными участниками всех последних Конгрессов по катализу являются И. Кожевников (Университет Ливерпуля, Великобритания), представивший устный доклад “Synthesis of chemicals from renewable feedstocks catalysed by polyoxometalates”, и Г. Яблонский (Университет Сен Луи, США) (на фото справа от В. Пармона). Слева от В. Пармона – В. Гальвита (Университет Гента, Бельгия), который вместе с Г. Яблонским и Г. Мариным является соавтором доклада “Newest evolution of the temporal analysis of products reactor system: TAP-3E combining catalyst synthesis with kinetic characterization”.

Помимо научной части были, конечно, и развлекательные программы, среди которых наибольшее впечатление произвел вечер в Баварском стиле в пивной в центре Мюнхена. Кроме баварского пива и закусок, участников ждали зажигательные баварские танцы вперемежку с демонстрацией рубки бревна по-баварски.
 За представлением внимательно следили участники Конгресса всех возрастов. В завершение все участники вечера, вслед за президентом Конгресса Й. Лерхером, присоединились к танцам вокруг всего зала.
За представлением внимательно следили участники Конгресса всех возрастов. В завершение все участники вечера, вслед за президентом Конгресса Й. Лерхером, присоединились к танцам вокруг всего зала.
 М. Синев (Институт химической физики им. Семенова, Москва) с внучкой на руках и Алекс Белл (Университет Беркли, Калифорния) обсуждают особенности баварского танцевального искусства, а Е. Козлова просто наслаждается зрелищем.
М. Синев (Институт химической физики им. Семенова, Москва) с внучкой на руках и Алекс Белл (Университет Беркли, Калифорния) обсуждают особенности баварского танцевального искусства, а Е. Козлова просто наслаждается зрелищем.
В целом 15-й Конгресс по катализу был несомненно полезен для обмена мнениями ученых, активно работающих в данной области, ознакомления с последними тенденциями и достижениями, для возобновления старых контактов и установления новых. При четкой организации работы Конгресса некоторое неудобство и неудовлетворенность вызвало размещение всех тезисов только на сайте Дехема в течение ограниченного периода, без предоставления участникам печатных либо электронных версий. По всей видимости, такое новшество не стоит рекомендовать в качестве примера для других международных конференций.
Ну, а после закрытия Конгресса российские и бывшие российские участники Конгресса взяли себе на память вывеску Конгресса.

Материал готовили В. Садыков, И. Симакова, В. Каичев, Е. Козлова,А. Степанов (ИК СО РАН, Новосибирск)
и все участники из ИППУ СО РАН (Омск) под руководством В.А. Лихолобова
3-7 декабря 2012 г.
Люксембург

3-7 декабря 2012 года в Люксембурге проведена XX Международная конференция по химическим реакторам ХИМРЕАКТОР-20. В 2012 году конференция имела статус юбилейной, она проводилась в двадцатый раз. История организации конференции уходит в 60-е годы прошлого столетия. Идея проведения научных мероприятий в области химической технологии, которые впоследствии переросли в Международную конференцию ХИМРЕАКТОР, принадлежит ее основателю, в прошлом сотруднику Института катализа Сибирского отделения РАН, Михаилу Гавриловичу Слинько. Он был бессменным председателем Программного комитета конференции, в последние годы его жизни – почетным председателем. В 2008 году Программным комитетом конференции была учреждена почетная лекция памяти профессора М.Г. Слинько. С тех пор она звучит на каждой конференции. Право чтения лекции по направлениям научной деятельности М.Г. Слинько предоставляется известным ученым мирового уровня, работающим в области химической технологии.

Выбором кандидатуры занимается Международный Программный комитет. Для представления лекции на юбилейной конференции ХИМРЕАКТОР после широкого обсуждения членами Программного комитета из 15-ти стран мира выбор пал на профессора Анджея Станкевича (Delft University of Technology, The Netherlands). Его лекция “From nanopinball to nanosnooker: towards perfect chemical reactors via fundamental concepts of process intensification” была посвящена развитию идей М.Г. Слинько в области математического моделирования каталитических процессов и конструирования реакторов. Профессор Станкевич продемонстрировал новые подходы к решению этих вопросов, в частности, он описал современную концепцию разработки химических реакторов, основанную на одновременном учете многих разномасштабных составляющих – от термодинамики и фундаментальных подходов к изучению химических реакций до функциональных и динамических аспектов. Лекция продемонстрировала примеры из практики создания сотрудниками Технического Университета Дельфта различных реакторов, в том числе использующих лазерные и электрические поля для контроля и регулирования молекулярной ориентации, а также точечного использования электромагнитного излучения для молекулярной активации. В заключение профессор Станкевич подчеркнул, что М.Г. Слинько первым сформулировал основы математического и компьютерного моделирования каталитических процессов и реакторов. Современные поиски путей к созданию новых реакторов построены на этом наследии, и они расширяются за счет применения физического моделирования энергетических полей и тепло- и массопереноса как основы для разработки новых, альтернативных концепций.
Высокий уровень пленарной сессии является отличительной чертой конференции ХИМРЕАКТОР. Известнейший специалист в области химической технологии профессор Джильберт Фрома (Texas A&M University, USA) отразил достижения в кинетическом моделировании процессов конверсии углеводородов. Профессор Джиан-Пьер Гилсон (Dalian Institute of Chemical Physics, Chinese Academy of Sci-ences, China) одновременно возглавляет Лабораторию катализа и спектрометрии (LCS) в городе Кан во Франции и является одним из крупнейших в мире специалистов в области нефтехимии. Его лекция была посвящена достижениям в области инжиниринга и катализа в процессах нефтепереработки. Лекция Председателя Международного Программного комитета конференции ХИМРЕАКТОР-20, директора Института катализа им. Г.К. Борескова Сибирского отделения Российской Академии наук, академика В.Н. Пармона была посвящена инженерным аспектам фотокаталитических процессов. Пленарный лектор профессор McKenna из Химико-технологического отделения Королевского Университета Канады (Queen’s University, Kingston, Canada), работающий в настоящее время в Университете Лиона во Франции, специализируется в области решения инновационных проектов в процессах полимеризации. В своей лекции “Olefin polymerisation reactors: what kind of problems do we face in olefin polymerisation reactors, and what kind of lab tools can we use to study them?” он задался вопросом – какие проблемы возникают в реакторах для полимеризации олефинов и какой инструментарий можно использовать для их изучения.
 Известный в мире работами в области биотехнологий профессор
Известный в мире работами в области биотехнологий профессор
Участники конференции представляли 35 стран мира. Научная программа мероприятия включала 7 пленарных лекций, 3 ключевых доклада, 60 устных докладов и 90 стендовых докладов. Тематика представленных на конференции докладов была традиционно широкой и затрагивала буквально все аспекты разработки, оптимизации и применения химических реакторов по всей глубине спектра исследований – от фундаментальных основ осуществления реакционных процессов до вопросов их эксплуатации в промышленности.
 Нельзя не отметить, что конференция, в силу ее систематического проведения раз в два года, имеет определённое количество участников, посещающих ее неоднократно. Поэтому встречи друзей и коллег по работе в различных городах мира придают конференции теплую окраску, создают хорошее настроение, способствуют радостной и доброжелательной атмосфере. Организаторы всегда предлагают участникам не только научные дискуссии, но и возможность пообщаться в неформальной обстановке, ознакомиться с достопримечательностями местности. Учитывая, что конференция ХИМРЕАКТОР-20 проходила в Люксембурге, который географически граничит со многими странами, участники имели возможность в рамках конференции и по ее окончании побывать в городах французской Лотарингии, в немецком Трире, в прекрасном городке Брюгге в Бельгии, провести совместный вечер в легендарном Шенгене, и конечно, познакомиться со старинными районами Люксембурга.
Нельзя не отметить, что конференция, в силу ее систематического проведения раз в два года, имеет определённое количество участников, посещающих ее неоднократно. Поэтому встречи друзей и коллег по работе в различных городах мира придают конференции теплую окраску, создают хорошее настроение, способствуют радостной и доброжелательной атмосфере. Организаторы всегда предлагают участникам не только научные дискуссии, но и возможность пообщаться в неформальной обстановке, ознакомиться с достопримечательностями местности. Учитывая, что конференция ХИМРЕАКТОР-20 проходила в Люксембурге, который географически граничит со многими странами, участники имели возможность в рамках конференции и по ее окончании побывать в городах французской Лотарингии, в немецком Трире, в прекрасном городке Брюгге в Бельгии, провести совместный вечер в легендарном Шенгене, и конечно, познакомиться со старинными районами Люксембурга.
Институт катализа им. Г.К. Борескова СО РАН планирует в 2014 году проведение XXI Международной конференции по химическим реакторам ХИМРЕАКТОР-21, которая будет посвящена 100-летию со дня рождения ее основателя, профессора Михаила Гавриловича Слинько.
Материал подготовлен Т. Замулиной и А. Загоруйко (ИК СО РАН, Новосибирск)
Materials: Acid’s surface residue, not just the catalyst, should get credit
By Mitch Jacoby
Titanium dioxide’s knack for mediating chemical reactions upon exposure to light has made the material a popular photocatalyst for various applications, such as sterilizing glass and other surfaces and splitting water to make hydrogen.
Researchers reported a few years ago that using hydrofluoric acid to synthesize nanocrystalline TiO2 significantly boosts its catalytic activity by exposing the material’s most active crystal faces. But a study just published in ACS Catalysis indicates the enhanced activity is mainly due to residual HF, not any particulars of crystal faceting (2013, DOI: 10.1021/cs400216a).
A crystal’s faces may be identical chemically, but structural and electronic differences can lead to differences in surface energy and catalytic activity. TiO2’s so-called (001) face, for example, is known to be more active than other TiO2 faces. For that reason, researchers commonly use HF to synthesize TiO2 because the acid yields a large fraction of (001)-faceted crystals.
Liqiang Jing of Heilongjiang University in China and coworkers confirmed via X-ray diffraction that increasing HF concentration during synthesis increases the fraction of crystals exhibiting (001) facets. And in degradation tests using acetaldehyde and phenol as model pollutants, the team confirmed that the most active TiO2 catalysts are indeed the ones with primarily exposed (001) faces. However, when the group removed surface fluoride—confirmed by surface analyses—catalytic activity plummeted despite the large fraction of (001) facets. The group proposes that surface-bound HF greatly enhances adsorption of O2 molecules, which capture electrons liberated by light and are thereby stimulated to react.
Can Li, a research director at China’s Dalian Institute of Chemical Physics and an expert in this area, says the work is convincing. And Baibiao Huang of Shandong University in China notes that the finding may be applicable to other oxide semiconductor photocatalysts with high-energy surfaces. “For that reason, it is of great significance,” he says.
ACS Meeting News: Catalytic process creates butanol as a better biofuel option for gasoline
By Stephen K. Ritter
 Ethanol derived from corn and sugarcane has been a blessing when it comes to transportation fuels. The millions of gallons of ethanol added to gasoline in the U.S. each year help oxygenate the fuel to limit pollution and stretch petroleum supplies. One draw-back is that ethanol reduces the energy content of gasoline, resulting in fewer miles per gallon when you are on the road.
Ethanol derived from corn and sugarcane has been a blessing when it comes to transportation fuels. The millions of gallons of ethanol added to gasoline in the U.S. each year help oxygenate the fuel to limit pollution and stretch petroleum supplies. One draw-back is that ethanol reduces the energy content of gasoline, resulting in fewer miles per gallon when you are on the road.
Chemists and chemical engineers who are at work to find improved biofuels have decided that butanol is a better option than ethanol. The researchers are now trying to settle on the best way to mass produce butanol to start replacing ethanol.
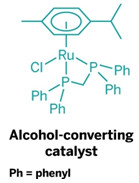
One solution offered by Duncan F. Wass and his research team at the University of Bristol, in England, is a new family of ruthenium catalysts that readily convert ethanol to butanol. Wass presented his group’s latest results during a session in the Division of Catalysis Science & Technology at the American Chemical Society national meeting this week in New Orleans.
Butanol with two additional carbons has about 30% higher energy content per gallon than ethanol, Wass explained. He noted that efforts are under way in the Midwest to retrofit some ethanol facilities to butanol production. But with his group’s new catalysts, ethanol facilities wouldn’t need to be altered—the ethanol produced in the facilities could simply be upgraded to butanol in an additional condensation reaction step.
“Our technology is an indirect path to butanol,” Wass told C&EN. “But it’s flexible because it can upgrade ethanol made from either petroleum or biomass.” The ruthenium catalysts are very efficient, he added, producing butanol with 95% selectivity and ethanol conversion of better than 40%.
The ethanol-to-butanol catalytic process is complementary to the fermentation of sugars directly into butanol using engineered microbes, which is being developed by other scientists, Wass noted. But fermentation to butanol has a few limitations, including low conversion of around 4 to 5% because of the inherent toxicity of butanol to the microorganisms.
The Bristol catalytic process has been patented, and Wass is working with scientists at BP Biofuels to develop the technology. “The new catalysts, while at an early stage, have much in common with modern petrochemical processing,” commented Ian Dobson of Butamax Advanced Biofuels, a joint venture created by BP and DuPont, and technology adviser to BP Biofuels. “The catalysts hold the prospect of being able to convert ethanol to butanol in high yield and at large scale.”
Solar Energy: The antireflective layer could lead to efficient, low-cost devices
By Prachi Patel
Adding a layer of graphene to silicon solar cells cuts the devices’ potential costs. Such graphene-silicon cells, though, convert light to electricity with low efficiencies. A team of researchers now has boosted this efficiency by giving the solar cells an antireflective veneer (Nano Lett., DOI: 10.1021/nl400353f).
Commercially available silicon solar cells convert about 15% of incoming light energy into electrical energy. But the cells are expensive to fabricate in part because of the costs of adding a thin, charge-carrying silicon layer on top, a process that requires high temperatures. The devices are also capped with a grid of thin silver wires that serve as an electrode.
Blue Hue
A 65-nm-thick titanium dioxide layer coats a graphene-silicon solar cell. The cell appears blue, because the coating has reduced visible light reflection from the silicon surface.
 Researchers led by Anyuan Cao of Peking University, in China, recently found that a graphene film can replace this charge-carrying layer, as well as serve as electrodes for the devices. Creating such films is a relatively inexpensive process. However, those graphene-silicon devices have efficiencies that top out around 8.6%, less than the 10% considered viable for commercial cells.
Researchers led by Anyuan Cao of Peking University, in China, recently found that a graphene film can replace this charge-carrying layer, as well as serve as electrodes for the devices. Creating such films is a relatively inexpensive process. However, those graphene-silicon devices have efficiencies that top out around 8.6%, less than the 10% considered viable for commercial cells.
So Cao and his colleagues, including Hongbian Li of the National Center for Nanoscience and Technology, in Beijing, and Hongwei Zhu, of Tsinghua University, turned to an old trick used to improve the efficiency of silicon solar cells. They coated the graphene-silicon structure with a 65-nm-thick layer of titanium dioxide. This coating decreased the amount of visible light reflected from the silicon surface from over 30% to less than 10%. Less re-flected light meant the device absorbed more light to convert to electricity, and the efficiency of the cells jumped to 14.6%.
The researchers currently grow the graphene layer on copper foil using chemical vapor deposition, which requires relatively high temperatures, and then transfer it to the top of a silicon wafer. Cao points out that they could further lower costs for the cells by painting or spaying a graphene solution onto the silicon wafers to produce the graphene film.
The graphene-silicon cells are several square millimeters in area. To be more practical, the devices need to be larger, Cao says, which presents another hurdle his team must face. As the area of a graphene film increases, so does its resistance, making the solar cells less efficient.
The researchers also need to make the devices more stable, says Yongsheng Chen, a chemistry professor at Nankai University, in China: The device efficiency drops after sitting in the air for 20 days. Nevertheless, he says that the work is significant for its use of two abundant materials—silicon and graphene—to make solar cells without the need for high-temperature processing. “This, combined with the high power-conversion efficiency, makes it potentially attractive for next-generation solar cell technology.”
Materials Science: Chemists push the bounds of what’s structurally possible in polymer architectures
By Stephen K. Ritter
 Polymer structures have traditionally been relatively simple, usually a linear chain made from a single type of monomer or perhaps a copolymer chain made from two or three types of monomers. Sometimes chemists might graft a side chain onto the polymer or cross-link the chains to form polymer networks.
Polymer structures have traditionally been relatively simple, usually a linear chain made from a single type of monomer or perhaps a copolymer chain made from two or three types of monomers. Sometimes chemists might graft a side chain onto the polymer or cross-link the chains to form polymer networks.
But that old simplicity is falling by the wayside. Advances in polymer synthetic techniques that allow better control over the size and shape of polymers are allowing researchers to think more like architects to dream up exotic new polymer designs. One goal of the work is to create macromolecules with functional properties for drug delivery, catalysis, chemical sensing, and other applications.
Several polymer science experts have now pooled their talents to find out how far they can push the limits of polymer architecture (J. Am. Chem. Soc., DOI: 10.1021/ja400890v).
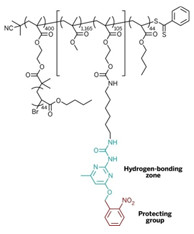
The research team is led by E. W. (Bert) Meijer of Eindhoven University of Technology, in the Netherlands; Krzysztof Matyjaszewski of Carnegie Mellon University; and Sergei S. Sheiko of the University of North Carolina, Chapel Hill. As a first test, the researchers set out to make a mega-sized copolymer consisting of a bottlebrush polymer with a collapsible tail, combining two of the recently developed advanced structural features in macromolecules.
Bottlebrush polymers are cylindrical in shape and have densely spaced side chains that resemble bristles on a brush. As for the polymer tail, chemists have created polymers with hydrogen-bonding capabilities that reversibly crumple into nanoparticles, a process similar to protein folding.
The team built the copolymer in four steps from methacrylate-based monomers. The process included grafting side chains onto the polymer and a postpolymerization step in which they added a hydrogen-bonding segment to a tail-like side chain. When the researchers shine ultraviolet light on the polymer, the tail sheds a protecting group to expose the hydrogen-bonding units. The end result is an unprecedented bottlebrush polymer with appended nanoparticles.
“This is an amazing example of how precision polymer synthesis techniques can be applied to the preparation of remarkably complex and functional macromolecular architectures,” says Marc A. Hillmyer, director of the University of Minnesota’s Center for Sustainable Polymers. “Combining controlled radical polymerizations, block copolymers, postpolymerization modifications, and triggered hydrogen-bonding interactions certainly pushes the limits of polymer synthesis.”
“A major challenge with synthetic polymers is that they are not as smart as well-defined natural systems, such as proteins,” says Craig J. Hawker, director of the Materials Research Laboratory at the University of California, Santa Barbara. “They do not fold in controllable ways or give unique molecular objects based on their synthetic design. I am convinced this work will spur other researchers to further push the frontiers of polymer synthesis, the ultimate goal being to design synthetic materials with many of the capabilities and properties of natural materials.”
Chemical & Engineering News
2nd International Conference CATALYSIS FOR RENEWABLE SOURCES:
FUEL, ENERGY, CHEMICALS
July 22-28, 2013
Lund, Sweden
http://conf.nsc.ru/CRS-2
The main objective of the conference is to increase the awareness of the achievements in the development of catalytic technologies for renewable sources processing, and to implement this knowledge for the new applications at the crossroads between different technologies.
INTERNATIONAL SCIENTIFIC COMMITTEE
Valentin N. Parmon, Chairman, Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
Dmitry Yu. Murzin, Vice-Chairman, Ǻbo Akademi University, Turku, Finland
Donato A.G. Aranda, Federal University of Rio de Janeiro, Brazil
Vasily A. Babkin, Favorsky Irkutsk Institute of Chemistry SB RAS, Irkutsk, Russia
Anthony Bridgwater, Aston University, UK
Jamal Chaouki, Polytechnique Montréal, Canada
Raghunath V. Chaudhari, University of Kansas, USA
Buxing Han, Institute of Chemistry CAS, Beijing, China
Erik Heeres, University of Groningen, The Netherlands
Can Li, Dalian Institute of Chemical Physics, CAS, China
Jyri-Pekka Mikkola, Umeå University, Sweden
Alexander S. Noskov, Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
Martyn Poliakoff, The University of Nottingham, UK
Parasuraman Selvam, Indian Institute of Technology, India
Sergei Varfolomeev, Emanuel Institute of Biochemical Physics RAS, Russia
Victor Zaichenko, Joint Institute of High Temperatures, Moscow, Russia
Vadim Yakovlev, Chairman of the Organizing Committee,
Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
SCIENTIFIC PROGRAM
Scientific program will comprise plenary invited lectures (1 hour), key-note presentations (30 minutes), oral presentations (20 minutes) and posters.
The official language of the conference is English.
PLENARY LECTURES
Professor Jan Brandin
Linné University, Kalmar & Växjö, Sweden
Professor Erik Heeres
University of Groningen, The Netherlands
Professor Simoni Margareti Plentz Meneghetti
Federal University of Alagoas, Institute of Chemistry
and Biotechnology, City of Maceió, Brazil
Professor Vladislav Sadykov
Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
Professor Tapio Salmi
Åbo Akademi, Turku, Finland
Dr. Juan Carlos Serrano-Ruiz
Abengoa Research, Seville, Spain
Professor Sergei Varfolomeev
Emanuel Institute of Biochemical Physics RAS, Moscow, Russia
KEY-NOTE LECTURES
Francesco Frusteri, Catia Cannilla, Giuseppe Bonura
CATALYTIC PRODUCTION OF OXYGENATED ADDITIVES BY GLYCEROL ETHERIFICATION
Institute CNR-ITAE “Nicola Giordano, Messina, Italy
Christian Hulteberg, Jan Brandin, Andreas Leveau
SELECTIVE CATALYSTS FOR GLYCEROL DEHYDRATION
Lund University, Faculty of Engineering, Sweden
B. Kuznetsov, V.I. Sharypov, S.A. Kuznetsova, S.V. Baryshnikov, N.V. Garyntseva
HETEROGENEOUS CATALYSIS METHODS FOR LIGNIN VALORIZATION INTO CHEMICALS AND ACTIVE CARBONS
Institute of Chemistry and Chemical Technology SB RAS, Krasnoyarsk, Russia
Mario Roberto Meneghetti
SIMULTANEOUS CONVERSION OF TRIACYLGLYCERIDES AND FATTY ACIDS INTO FATTY ACID METHYL ESTERS USING ORGANOMETALLIC CATALYSTS
Federal University of Alagoas, Group of Catalysis and Chemical Reactivity, City of Maceió, Brazil
Mani Subramanian
NEW GENETIC INSIGHTS TO CONSIDER COFFEE WASTE AS A FEEDSTOCK FOR FUEL, FEED AND CHEMICALS
Center for Biocatalysis & Bioprocessing (CBB), Coralville, Iowa, USA
Important dates
| June 5 | Distribution of the preliminary program |
| June 20 | Confirmation of participation, fee payment |
| July 1 | Distribution of the final program |
| Luly 21 | On site Registration |
| July 22 | Conference opening |
Contacts
CRS-2 Conference Secretariat:
Tatiana Zamulina, Marina Suvorova
zam@catalysis.ru; suvorova@catalysis.ru
Boreskov Institute of Catalysis SB RAS
pr. Akademika Lavrentieva, 5, 630090 Novosibirsk, Russia
Tel.: +7 383 326 9536; +7 383 326 9584; Fax: +7 383 330 8056

| Международная конференция http: //conf.nsc.ru/conf-tashkent-2013 |
Международная конференция “Каталитические процессы нефтепереработки, нефтехимии и экологии” состоится с 15 по 16 октября 2013 г. в столице Республики Узбекистан г. Ташкенте.
Организаторы конференции
НАУЧНАЯ ПРОГРАММА
Международная конференция “Каталитические процессы нефтепереработки, нефтехимии и экологии” позволит рассмотреть современное состояние научно-исследовательских и практически значимых работ по актуальным направлениям:
В научную программу будут включены 8 приглашенных лекций ведущих специалистов (30 мин), 25 устных докладов (20 мин и 10 мин) и стендовые доклады.
Планируется, что в работе конференции примут участие специалисты из Азербайджана, Армении, Белоруссии Казахстана, России, Узбекистана и Украины.
Участники конференции получат возможность продемонстрировать и обсудить свои работы, определить перспективные направления научных исследований с учётом их развития в странах СНГ и обсудить возможности создания условий, способствующих взаимовыгодному сотрудничеству.
Рабочие языки конференции – русский, узбекский и английский.
Дополнительно Конференция предлагает дискуссии за Круглым столом, выставки-презентации компаний, деловые встречи с руководителями промышленных химических предприятий республики Узбекистан.
Международный программный комитет
Турабжанов С.М., профессор (Узбекистан) – Председатель
Пармон В.Н., академик РАН (Россия) – Председатель
Гончарук В.В., академик НАНУ (Украина)
Дадаходжаев А.Т., профессор (Узбекистан)
Досумов К.Д., профессор (Казахстан)
Исмагилов З.Р., член-корр. РАН (Россия)
Исмаилов Э., профессор (Азербайжан)
Капустин В.М., профессор (Россия)
Каюмов Ш.Ш., МВССО РУз (Узбекистан)
Лихолобов В.А., член-корр. РАН (Россия)
Лунин В.В., академик РАН (Россия)
Мажидов Ш.Х., НХК “Узбекнефтегаз” (Узбекистан)
Мазгаров А.М., академик АНРТ (Россия)
Махкамов Х.М., профессор (Узбекистан)
Носков А.С., профессор (Россия)
Парпиев О.Р., ККРНТ (Узбекистан)
Пимерзин А.А., профессор (Россия)
Рашидова С.Ш., академик АНРУз (Узбекистан)
Хаджиев С.Н., академик (Россия)
Юнусов М.П., профессор (Узбекистан)
Яруллин Р.С., “Татнефтехиминвест-холдинг” (Россия)
Организационный комитет
Турабжанов С.М., ТХТИ, Ташкент – Председатель
Носков А.С., ИК СО РАН, Новосибирск – Председатель
Адилов Р.И., ТХТИ, Ташкент
Белый А.С., ИППУ СО РАН, Омск
Гулямов Ш.Т., УзКФИТИ, Ташкент
Дадаходжаев А.Т., ГАК “УзХимпром”, Ташкент
Закиров Б.С., ИОНХ АНРУз, Ташкент
Зиёдуллаев О.Э., ТХТИ, Ташкент
Ибодуллаев А., ТХТИ, Ташкент
Икрамов А., ТХТИ, Ташкент
Климов О.В., ИК СО РАН, Новосибирск
Лавошников В.В., УзКФИТИ, Ташкент
Мансурова М.С., УзКФИТИ, Ташкент
Махкамов Х.М., УзКФИТИ, Ташкент
Насуллаев Х.А., УзКФИТИ, Ташкент
Рахмонбердиев Г., ТХТИ, Ташкент
Сайфуддинов Р., ТХТИ, Ташкент
Чумаченко В.А., ИК СО РАН, Новосибирск
Юнусов М.П., УзКФИТИ, Ташкент
Ключевые даты
| 10 сентября | – публикация научной программы на сайте |
| 14 октября | – день заезда |
| 15-16 октября | – рабочие дни |
| 17 октября | – отъезд участников (пост-тур) |
Ученые секретари Конференции
|
Россия: |
Узбекистан: Кадырова Дилором Салиховна |
Международная конференция “Каталитические процессы нефтепереработки, нефтехимии и экологии” Ташкент, Республика Узбекистан |
|
X Международная научно- техническая конференция “Энерго- и материало сберегающие экологически чистые технологии” Гродно, Республика Беларусь |
|
Asian Polyolefin Workshop (APO 2013) Beijing, China |
|
14th Tetrahedron Symposium (Asia Edition) “Challenges in Organic & Bioorganic Chemistry” Seoul, Republic of Korea |
|
II Всероссийская конференция “Методы исследования состава и структуры функциональных материалов” Новосибирск, Россия |
|
ХХVII Международная научно-техническая конференция “Химические реактивы, реагенты и процессы малотоннажной химии” (РЕАКТИВ-2013) Иркутск, Россия |
|
VI Международная научная конференция “Актуальные проблемы физики твердого тела” (ФТТ-2013) Минск, Республика Беларусь |
|
X Российская конференция молодых научных сотрудников и аспирантов “Физико-химия и технология неорганических материалов” Москва, Россия |
|
4th Asian Symposium on Advanced Materials: Chemistry, Physics & Biomedicine of Functional and Novel Materials” (ASAM-4) Taipei, Taiwan |
|
IV Российское совещание по органической минералогии (с международным участием) Черноголовка, Россия |
|
2013 International Conference on Frontiers of Environment, Energy and Bioscience (ICFEEB 2013) Beijing, China |
|
3rd European Conference on Environmental Applications of Advanced Oxidation Processes (EAAOP3 2013) Almería, Spain |
|
Международный химический форум в рамках Международной выставки “Химия 2013” Москва, Россия |
|
Международный симпозиум “Физика кристаллов 2013” Москва, Россия |
http://www.misis.ru/crystalxxi/tabid/3251/language/ru- |
IV Межотраслевая конференция “Вода в промышленности-2013” Москва, Россия |
|
Ежегодная конференция РХО им. Д.И. Менделеева “Ресурсо- и энергосберегающие технологии в химической и нефтехимической промышленности” Москва, Россия |
|
Carbon Dioxide Utilisation Summit 2013 Brussels, Belgium |
|
|
http://v11.vuturevx.com/exchange- |
V Молодежная научно- техническая конференция “Наукоемкие химические технологии-2013” Москва, Россия |
http://www.nanometer.ru/2013/06/09/ |
II Всероссийский симпозиум с участием иностранных ученых “Кинетика и динамика обменных процессов” Геленджик, Россия |
|
ACI’s 3rd Annual Gasification Summit London, UK |
|
3rd New Energy Forum Chengdu, China |
|
IX Санкт-Петербургская конференция молодых ученых с международным участием “Современные проблемы науки о полимерах” Cанкт-Петербург, Россия |
|
Научно-практическая конференция “Полимеризационные пластмассы – 2013: сырьевая база, производство и переработка” Cанкт-Петербург, Россия |
proekt@plastpolymer.com |
World Congress on Petrochemistry and Chemical Engineering San Antonio, USA |
http://www.omicsgroup.com/conferences/petrochemist |
XXXI Всероссийский симпозиум молодых ученых по химической кинетике Московская обл., Россия |
|
V Международный форум по “зелёной” химии и промышленным биотехнологиям (Грэйнтек-2013) Москва, Россия |
|
International Conference on the Advancement of Materials and Nanotechnology (ICAMN 2013) Penang, Malaysia |
|
IV Международная конференция “Наноразмерные системы: строение – свойства – технологии” (НАНСИС-2013) Киев, Украина |
http://www.nas.gov.ua/conferences/nansys2013/ |
Bioenergy Commodity Trading Brussels, Belgium |
|
III Всероссийская конференция c международным участием “Актуальные вопросы химической технологии и защиты окружающей среды” Новочебоксарск, Чувашская Республика, Россия |
|
II Международный научно- методологический семинар “Возобновляемые источники энергии. Потенциал возобновляемой энергии и перспективы его достижения” Минск, Республика Беларусь |
|
V Всероссийская научно- практическая конференция “Современные наукоемкие инновационные технологии” Самара, Россия |
|
World Elastomer Summit 2013 Lyon, France |
|
3rd Nano Today Conference (Nano Today 2013) Biopolis, Singapore |
|
2nd Workshop on Photocatalytic and Superhydrophilic Surfaces (PSS 2013) Manchester, UK |
|
XXII International Conference on Processing and Fabrication of Advanced Materials (PFAM XXII) Singapore |
|
International Conference on Agriculture Science and Environment Engineering (ICASEE 2013) Beijing, China |
|
|
|
|
2nd International Conference on Environmental Horizon “Greening the Blue” (ICEH-2014) Karachi, Pakistan |
|
1st International Symposium on Nanoparticles-Nanomaterials and Applications (ISN2A-2014) Lisbon, Portugal |
|
International Conference on Petroleum and Petrochemical Engineering (ICPPE 2014) Macau, China |
|
Materials Challenges In Alternative & Renewable Energy 2014 (MCARE 2014) Clearwater, FL, USA |
http://ceramics.org/meetings/materials-challenges-in- |
International Conference on Nanotechnology, Nanomaterials & Thin Films for Energy Applications London, UK |
|
3rd International Conference on Green Polymer Chemistry 2014 London, UK |
|
VIII Всероссийская конференция с международным участием молодых ученых по химии “Менделеев-2014” Санкт-Петербург, Россия |
|
III Всероссийская научная конференция с международным участием “Успехи синтеза и комплексообразования” Москва, Россия |
|
6-я Международная конференция “Платиновые металлы в современной индустрии, водородной энергетике и в сферах жизнеобеспечения будущего” Тель Авив, Израиль |
|
International conference on BioMass Florence, Italy |
|
Научно-технологический симпозиум “Нефтепереработка: катализаторы и гидропроцессы” Пушкин (Санкт-Петербург), Россия |
|
7th International Conference “Inverse Problems: Modeling and Simulation” Fethiye, Turkey |
|
7th Tokyo Conference on Advanced Catalytic Science and Technology (TOCAT7) Kyoto, Japan |
|
1st International Conference on Mechanics of Composites (MECHCOMP2014) Long Island, NY, USA |
|
4th International Colloids Conference “Surface Design & Engineering” Madrid, Spain |
|
21st World Petroleum Congress Moscow, Russia |
|
22nd European Biomass Conference and Exhibition (EU BC&E 2014) Hamburg, Germany |
|
International Conference “Modern Physical Chemistry for Advanced Materials” Kharkiv, Ukraine |
|
11th International Symposium “Scientific Bases for the Preparation of Heterogeneous Catalysts” Louvain-la-Neuve, Belgium |
|
11th Conference on Solid State Chemistry (SSC 2014) Trenčianske Teplice, Slovakia |
|
XII International Conference on Nanostructured Materials (NANO 2014) Moscow, Russia |
http://www.nano2014.org/ |
7th International Conference on Technological Advances of Thin Films & Surface Coatings (Thin Films 2014) Chongqing, China |
|
21st International Congress of Chemical and Process Engineering (CHISA 2014) Prague, Czech Republic |
|
23rd International Symposium on Chemical Reaction Engineering (ISCRE 23) Bangkok, Thailand |
|
International Summer School on Photovoltaics and New Concepts of Quantum Solar Energy Conversion (Quantsol) Hirschegg, Austria |
|
6th International FEZA Conference “Porous Systems: From Novel Materials to Sustainable Solutions” Leipzig, Germany |
|
III международная конференция стран СНГ “Золь-гель синтез и исследование неорганических соединений, гибридных функциональных материалов и дисперсных систем” Суздаль, Россия |
|
XXI International Conference on Chemical Reactors (CHEMREACTOR-21) Delft, The Netherlands |
|
12th International Conference on Fundamental and Applied Aspects of Physical Chemistry (Physical Chemistry 2014) Belgrade, Serbia |
|
II Российский конгресс по катализу “РОСКАТАЛИЗ” Самара, Россия |
|
3rd International Conference on Polymer Processing and Characterization (ICPPC-2014) Kottayam, Kerala, India |
|
|
|
|
18th International Conference on Composite Structures (ICCS18) Lisbon, Portugal |
|
XII European Congress on Catalysis (EuropaCat XII) Kazan, Russia |
|
European Symposium on Chemical Reaction Engineering (ESCRE 2015) Fürstenfeldbruck, Germany |
|
Уважаемые коллеги! Дорогие друзья!
Я приглашаю Вас стать членами авторского актива нового электронного научного журнала «Технологии добычи и использования углеводородов».
Журнал имеет своей главной целью информационную поддержку создания новых механизмов взаимодействия бизнеса, науки и государства в рамках Технологической платформы (ТП) «Технологии добычи и использования углеводородов», образованной по решению Правительственной комиссии по высоким технологиям и инновациям.
Издание направлено на информирование широкой целевой аудитории о реализации инновационной политики в области добычи и использования углеводородов: создания совокупности «прорывных» энергоэффективных и ресурсосберегающих технологий, формирующих новые рынки высокотехнологичной продукции и услуг в нефтегазодобыче, нефтегазовом машиностроении, транспортировке и эффективном использовании углеводородных ресурсов.
Журнал будет выходить ежеквартально на портале www.oilring.ru, созданном для формирования инновационной среды нефтегазовой отрасли в виртуальном пространстве.
Тематика сфокусирована на новейших технологиях, на технических инновациях и прорывных научных исследованиях. В поле зрения издания находятся, прежде всего, отечественные разработки. Особое внимание журнал намерен уделять молодым ученым, конструкторам, инженерам – будущему российской нефтегазовой мысли.
Стратегическое развитие электронного журнала направляет Редакционный совет, в состав которого входят видные ученые, руководители крупных компаний, представители Минэнерго России. Председатель Редакционного совета – академик РАН, директор Института проблем нефти и газа РАН А.Н. Дмитриевский. Главный редактор журнала – генеральный директор ОАО «Зарубежнефть» С.И. Кудряшов.
Каждый номер открывается рубрикой «Технология», в которой дается описание одной из новейших разработок. Рубрикация журнала охватывает практически полный спектр проблематики нефтегазовой отрасли. В поле зрения издания, прежде всего, отечественные разработки:
Обращаю Ваше внимание также на рубрики «Университет. Высшее образование», где будут публиковаться материалы о передовом опыте подготовки кадров для нефтегазовой отрасли и «Новые книги», где будет собираться информация об изданиях нефтегазовой тематики.
В самом ближайшем времени планируется включить электронный научный журнал «Технологии добычи и использования углеводородов» в перечень ведущих рецензируемых научных журналов и изданий, в которых должны быть опубликованы научные результаты диссертаций на соискание ученой степени доктора и кандидата наук, утвержденный Президиумом Высшей Аттестационной комиссии Министерства образования и науки Российской Федерации (ВАК), а также в базы системы Российского индекса научного цитирования.
Я предлагаю Вам присылать в адрес редакции content@oilring.ru результаты своих исследований, оформленные в виде научных статей. Требования к оформлению стандартные. Желательно в теме письма указать рубрику, для которой предназначена статья.
Первый номер журнала намечен к выпуску в июне 2013 года. Формирование редакционного портфеля для него будет завершено 10 июня 2013г.
Призываю Вас присоединиться к кругу авторов и читателей нового электронного научного журнала! За дополнительной информацией обращайтесь, пожалуйста, в нашу редакцию (oilring@oilring.ru).
Искренне Ваш,
Заместитель Председателя Редакционного совета электронного
научного журнала «Технологии добычи и использования углеводородов»,
Первый проректор по стратегическому развитию РГУ нефти и газа им. М.И. Губкина
Д.т.н., профессор М.А. Силин


